CaMKII-dependent SR Ca leak contributes to doxorubicin-induced impaired Ca handling in isolated cardiac myocytes
- PMID: 21819992
- PMCID: PMC3226826
- DOI: 10.1016/j.yjmcc.2011.07.016
CaMKII-dependent SR Ca leak contributes to doxorubicin-induced impaired Ca handling in isolated cardiac myocytes
Abstract
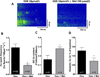
Doxorubicin (DOX) is one of the most effective chemotherapeutic agents, but cardiotoxicity limits DOX therapy. Although the mechanisms are not entirely understood, reactive oxygen species (ROS) appear to be involved in DOX cardiotoxicity. Ca/calmodulin dependent protein kinase II (CaMKII) can be activated by ROS through oxidation and is known to contribute to myocardial dysfunction through Ca leakage from the sarcoplasmic reticulum (SR). We hypothesized that CaMKII contributes to DOX-induced defects in intracellular Ca ([Ca](i)) handling. Cardiac myocytes were isolated from wild-type (WT) adult rat hearts and from mouse hearts lacking the predominant myocardial CaMKII isoform (CaMKIIδ(-/-), KO) vs. WT. Isolated cardiomyocytes were investigated 30 min after DOX (10 μmol/L) superfusion, using epifluorescence and confocal microscopy. Intracellular ROS-generation ([ROS](i)) and [Ca](i) handling properties were assessed. In a subset of experiments, KN-93 or AIP (each 1 μmol/L) were used to inhibit CaMKII. Melatonin (Mel, 100 μmol/L) served as ROS-scavenger. Western blots were performed to determine the amount of CaMKII phosphorylation and oxidation. DOX increased [ROS](i) and led to significant diastolic [Ca](i) overload in rat myocytes. This was associated with reduced [Ca](i) transients, a 5.8-fold increased diastolic SR Ca leak and diminished SR Ca content. ROS-scavenging partially rescued Ca handling. Western blots revealed increased CaMKII phosphorylation, but not CaMKII oxidation after DOX. Pharmacological CaMKII inhibition attenuated diastolic [Ca](i) overload after DOX superfusion and led to partially restored [Ca](i) transients and SR Ca content, presumably due to reduced Ca spark frequency. In line with this concept, isoform-specific CaMKIIδ-KO attenuated diastolic [Ca](i) overload and Ca spark frequency. DOX exposure induces CaMKII-dependent SR Ca leakage, which partially contributes to impaired cellular [Ca](i) homeostasis. Pharmacological and genetic CaMKII inhibition attenuated but did not completely abolish the effects of DOX on [Ca](i). In light of the clinical relevance of DOX, further investigations seem appropriate to determine if CaMKII inhibition could reduce DOX-induced cardiotoxicity.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures












Similar articles
-
Calcium/calmodulin-dependent protein kinase II contributes to cardiac arrhythmogenesis in heart failure.Circ Heart Fail. 2009 Nov;2(6):664-75. doi: 10.1161/CIRCHEARTFAILURE.109.865279. Epub 2009 Jul 31. Circ Heart Fail. 2009. PMID: 19919992 Free PMC article.
-
Reactive oxygen species-activated Ca/calmodulin kinase IIδ is required for late I(Na) augmentation leading to cellular Na and Ca overload.Circ Res. 2011 Mar 4;108(5):555-65. doi: 10.1161/CIRCRESAHA.110.221911. Epub 2011 Jan 20. Circ Res. 2011. PMID: 21252154 Free PMC article.
-
Enhanced late INa induces proarrhythmogenic SR Ca leak in a CaMKII-dependent manner.J Mol Cell Cardiol. 2014 Nov;76:94-105. doi: 10.1016/j.yjmcc.2014.08.016. Epub 2014 Aug 27. J Mol Cell Cardiol. 2014. PMID: 25173923
-
CaMKIIdelta overexpression in hypertrophy and heart failure: cellular consequences for excitation-contraction coupling.Braz J Med Biol Res. 2005 Sep;38(9):1293-302. doi: 10.1590/s0100-879x2005000900002. Epub 2005 Aug 26. Braz J Med Biol Res. 2005. PMID: 16138211 Review.
-
Role of oxidants on calcium and sodium movement in healthy and diseased cardiac myocytes.Free Radic Biol Med. 2013 Oct;63:338-49. doi: 10.1016/j.freeradbiomed.2013.05.035. Epub 2013 Jun 1. Free Radic Biol Med. 2013. PMID: 23732518 Review.
Cited by
-
An Overview of the Role of Calcium/Calmodulin-Dependent Protein Kinase in Cardiorenal Syndrome.Front Physiol. 2020 Jul 14;11:735. doi: 10.3389/fphys.2020.00735. eCollection 2020. Front Physiol. 2020. PMID: 32760284 Free PMC article. Review.
-
Cardiac side effects of anticancer treatments: new mechanistic insights.Curr Heart Fail Rep. 2012 Sep;9(3):211-8. doi: 10.1007/s11897-012-0098-4. Curr Heart Fail Rep. 2012. PMID: 22752360 Free PMC article. Review.
-
CaMKII is a nodal signal for multiple programmed cell death pathways in heart.J Mol Cell Cardiol. 2017 Feb;103:102-109. doi: 10.1016/j.yjmcc.2016.12.007. Epub 2016 Dec 24. J Mol Cell Cardiol. 2017. PMID: 28025046 Free PMC article. Review.
-
EPAC1 inhibition protects the heart from doxorubicin-induced toxicity.Elife. 2023 Aug 8;12:e83831. doi: 10.7554/eLife.83831. Elife. 2023. PMID: 37551870 Free PMC article.
-
Differential cardiotoxic electrocardiographic response to doxorubicin treatment in conscious versus anesthetized mice.Physiol Rep. 2021 Aug;9(15):e14987. doi: 10.14814/phy2.14987. Physiol Rep. 2021. PMID: 34337891 Free PMC article.
References
-
- Yeh ETH, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–2247. - PubMed
-
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229. - PubMed
-
- Weiss RB. The anthracyclines: will we ever find a better doxorubicin? Semin Oncol. 1992;19:670–686. - PubMed
-
- Zuppinger C, Timolati F, Suter T. Pathophysiology and diagnosis of cancer drug induced cardiomyopathy. Cardiovas Toxicol. 2007;7:61–66. - PubMed
-
- Wouters KA, Kremer LC, Miller TL, Herman EH, Lipshultz SE. Protecting against anthracycline-induced myocardial damage: a review of the most promising strategies. Br J Haematol. 2005;131:561–578. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

