Role of VPO1, a newly identified heme-containing peroxidase, in ox-LDL induced endothelial cell apoptosis
- PMID: 21820048
- PMCID: PMC3570029
- DOI: 10.1016/j.freeradbiomed.2011.07.004
Role of VPO1, a newly identified heme-containing peroxidase, in ox-LDL induced endothelial cell apoptosis
Abstract
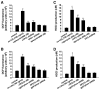
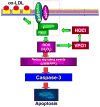
Myeloperoxidase (MPO) is an important enzyme involved in the genesis and development of atherosclerosis. Vascular peroxidase 1 (VPO1) is a newly discovered member of the peroxidase family that is mainly expressed in vascular endothelial cells and smooth muscle cells and has structural characteristics and biological activity similar to those of MPO. Our specific aims were to explore the effects of VPO1 on endothelial cell apoptosis induced by oxidized low-density lipoprotein (ox-LDL) and the underlying mechanisms. The results showed that ox-LDL induced endothelial cell apoptosis and the expression of VPO1 in endothelial cells in a concentration- and time-dependent manner concomitant with increased intracellular reactive oxygen species (ROS) and hypochlorous acid (HOCl) generation, and up-regulated protein expression of the NADPH oxidase gp91(phox) subunit and phosphorylation of p38 MAPK. All these effects of ox-LDL were inhibited by VPO1 gene silencing and NADPH oxidase gp91(phox) subunit gene silencing or by pretreatment with the NADPH oxidase inhibitor apocynin or diphenyliodonium. The p38 MAPK inhibitor SB203580 or the caspase-3 inhibitor DEVD-CHO significantly inhibited ox-LDL-induced endothelial cell apoptosis, but had no effect on intracellular ROS and HOCl generation or the expression of NADPH oxidase gp91(phox) subunit or VPO1. Collectively, these findings suggest for the first time that VPO1 plays a critical role in ox-LDL-induced endothelial cell apoptosis and that there is a positive feedback loop between VPO1/HOCl and the now-accepted dogma that the NADPH oxidase/ROS/p38 MAPK/caspase-3 pathway is involved in ox-LDL-induced endothelial cell apoptosis.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




References
-
- Malle E, Waeg G, Schreiber R, Gröne EF, Sattler W, Gröne HJ. Immunohistochemical evidence for the myeloperoxidase/H2O2/halide system in human atherosclerotic lesions. Eur J Biochem. 2000;267:4495–4503. - PubMed
-
- Podrez EA, Abu-Soud HM, Hazen SL. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radic Biol Med. 2000;28:1717–1725. - PubMed
-
- Stocker R, Huang A, Jeranian E, Hou JY, Wu TT, Thomas SR, Keaney JF., Jr Hypochlorous acid impairs endothelium-derived nitric oxide bioactivity through a superoxide-dependent mechanism. Arterioscler Thromb Vasc Biol. 2004;24:2028–2033. - PubMed
-
- Malle E, Marsche G, Arnhold J, Davies MJ. Modification of low-density lipoprotein by myeloperoxidase-derived oxidants and reagent hypochlorous acid. Biochim Biophys Acta. 2006;1761:392–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

