Changes of chondrocyte expression profiles in human MSC aggregates in the presence of PEG microspheres and TGF-β3
- PMID: 21820171
- PMCID: PMC3176960
- DOI: 10.1016/j.biomaterials.2011.07.056
Changes of chondrocyte expression profiles in human MSC aggregates in the presence of PEG microspheres and TGF-β3
Abstract
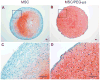
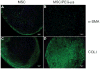
Biomaterial microparticles are commonly utilized as growth factor delivery vehicles to induce chondrogenic differentiation of mesenchymal stem/stromal cells (MSCs). To address whether the presence of microparticles could themselves affect differentiation of MSCs, a 3D co-aggregate system was developed containing an equal volume of human primary bone marrow-derived MSCs and non-degradable RGD-conjugated poly(ethylene glycol) microspheres (PEG-μs). Following TGF-β3 induction, differences in cell phenotype, gene expression and protein localization patterns were found when compared to MSC aggregate cultures devoid of PEG-μs. An outer fibrous layer always found in differentiated MSC aggregate cultures was not formed in the presence of PEG-μs. Type II collagen protein was synthesized by cells in both culture systems, although increased levels of the long (embryonic) procollagen isoforms were found in MSC/PEG-μs aggregates. Ubiquitous deposition of type I and type X collagen proteins was found in MSC/PEG-μs cultures while the expression patterns of these collagens was restricted to specific areas in MSC aggregates. These findings show that MSCs respond differently to TGF-β3 when in a PEG-μs environment due to effects of cell dilution, altered growth factor diffusion and/or cellular interactions with the microspheres. Although not all of the expression patterns pointed toward improved chondrogenic differentiation in the MSC/PEG-μs cultures, the surprisingly large impact of the microparticles themselves should be considered when designing drug delivery/scaffold strategies.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures



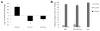
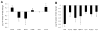
References
-
- Ulrich-Vinther M, Maloney MD, Schwarz EM, Rosier R, O’Keefe RJ. Articular cartilage biology. J Am Acad Orthop Surg. 2003;11:421–30. - PubMed
-
- Daher RJ, Chahine NO, Greenberg AS, Sgaglione NA, Grande DA. New methods to diagnose and treat cartilage degeneration. Nat Rev Rheumatol. 2009;5:599–607. - PubMed
-
- Nelson L, Fairclough J, Archer CW. Use of stem cells in the biological repair of articular cartilage. Expert Opin Biol Th. 2010;10:43–55. - PubMed
-
- Keeney M, Lai JH, Yang F. Recent progress in cartilage tissue engineering. Curr Opin Biotechnol. 2011 doi: 10.1016/j.copbio.2011.04.003. Available from URL: http://www.ncbi.nlm.nih.gov/pubmed/21531126. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

