Targeting Trypanosoma cruzi sterol 14α-demethylase (CYP51)
- PMID: 21820552
- PMCID: PMC3488290
- DOI: 10.1016/B978-0-12-385863-4.00004-6
Targeting Trypanosoma cruzi sterol 14α-demethylase (CYP51)
Abstract
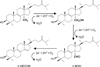
There are at least two obvious features that must be considered upon targeting specific metabolic pathways/enzymes for drug development: the pathway must be essential and the enzyme must allow the design of pharmacologically useful inhibitors. Here, we describe Trypanosoma cruzi sterol 14α-demethylase as a promising target for anti-Chagasic chemotherapy. The use of anti-fungal azoles, which block sterol biosynthesis and therefore membrane formation in fungi, against the protozoan parasite has turned out to be highly successful: a broad spectrum anti-fungal drug, the triazole compound posaconazole, is now entering phase II clinical trials for treatment of Chagas disease. This review summarizes comparative information on anti-fungal azoles and novel inhibitory scaffolds selective for Trypanosomatidae sterol 14α-demethylase through the lens of recent structure/functional characterization of the target enzyme. We believe our studies open wide opportunities for rational design of novel, pathogen-specific and therefore more potent and efficient anti-trypanosomal drugs.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures





References
-
- Aoyama Y, Yoshida Y, Sonoda Y, Sato Y. 7-Oxo-24,25-dihydrolanosterol: a novel lanosterol 14 alpha-demethylase (P-45014DM) inhibitor which blocks electron transfer to the oxyferro intermediate. Biochim. Biophys. Acta. 1987;922:270–277. - PubMed
-
- Apt W, Aguilera X, Arribada A, Perez C, Miranda C, Sanchez G, et al. Treatment of chronic Chagas’ disease with itraconazole and allopurinol. Am. J. Trop. Med. Hyg. 1998;59:133–138. - PubMed
-
- Araujo MS, Martins-Filho OA, Pereira ME, Brener Z. A combination of benznidazole and ketoconazole enhances efficacy of chemotherapy of experimental Chagas’ disease. J. Antimicrob. Chemother. 2000;45:819–824. - PubMed
-
- Beach DH, Goad LJ, Holz GG., Jr Effects of ketoconazole on sterol biosynthesis by Trypanosoma cruzi epimastigotes. Biochem. Biophys. Res. Commun. 1986;136:851–856. - PubMed
-
- Benveniste P. Sterol biosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1986;37:275–308.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

