Crosstalk between EET and HO-1 downregulates Bach1 and adipogenic marker expression in mesenchymal stem cell derived adipocytes
- PMID: 21821145
- PMCID: PMC3645487
- DOI: 10.1016/j.prostaglandins.2011.07.005
Crosstalk between EET and HO-1 downregulates Bach1 and adipogenic marker expression in mesenchymal stem cell derived adipocytes
Abstract
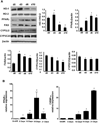
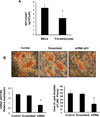
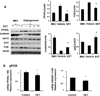
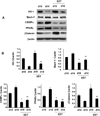
Epoxygenase activity and synthesis of epoxyeicosatrienoic acids (EETs) have emerged as important modulators of obesity and diabetes. We examined the effect of the EET-agonist 12-(3-hexylureido)dodec-8(2) enoic acid on mesenchymal stem cell (MSC) derived adipocytes proliferation and differentiation. MSCs expressed substantial levels of EETs and inhibition of soluble epoxide hydrolase (sEH) increased the level of EETs and decreased adipogenesis. EET agonist treatment increased HO-1 expression by inhibiting a negative regulator of HO-1 expression, Bach-1. EET treatment also increased βcatenin and pACC levels while decreasing PPARγ C/EBPα and fatty acid synthase levels. These changes were manifested by a decrease in the number of large inflammatory adipocytes, TNFα, IFNγ and IL-1α, but an increase in small adipocytes and in adiponectin levels. In summary, EET agonist treatment inhibits adipogenesis and decreases the levels of inflammatory cytokines suggesting the potential action of EETs as intracellular lipid signaling modulators of adipogenesis and adiponectin.
Copyright © 2011. Published by Elsevier Inc.
Figures

References
-
- Abraham NG, Kappas A. Pharmacological. clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. - PubMed
-
- Abraham NG. Molecular regulation—biological role of heme in hematopoiesis. Blood Rev. 1991;5:19–28. - PubMed
-
- Chertkov JL, Jiang S, Lutton JD, Levere RD, Abraham NG. Hemin stimulation of hemopoiesis in murine long-term bone marrow culture. Exp Hematol. 1991;19:905–909. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

