The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings
- PMID: 21821475
- PMCID: PMC5528309
- DOI: 10.1016/j.molonc.2011.07.001
The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings
Abstract
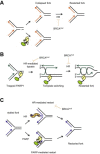
Poly (ADP-ribose) polymerase (PARP) inhibitors effectively kill tumours defective in the BRCA1 or BRCA2 genes through the concept of synthetic lethality. It is suggested that PARP inhibitors cause an increase in DNA single-strand breaks (SSBs), which are converted during replication to irreparable toxic DNA double-strand breaks (DSBs) in BRCA1/2 defective cells. There are a number of recent reports challenging this model. Here, alternative models that are not mutually exclusive are presented to explain the synthetic lethality between BRCA1/2 and PARP inhibitors. One such model proposes that PARP inhibition causes PARP-1 to be trapped onto DNA repair intermediates, especially during base excision repair. This may in turn cause obstruction to replication forks, which require BRCA-dependent homologous recombination to be resolved. In another model, PARP is directly involved in catalysing replication repair in a distinct pathway from homologous recombination. Experimental evidence supporting these novel models to explain the PARP-BRCA synthetic lethality are discussed.
Copyright © 2011 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Allinson, S.L. , Dianova, Dianov, G.L. , 2003. Poly(ADP-ribose) polymerase in base excision repair: always engaged, but not essential for DNA damage processing. Acta Biochim. Pol. 50, 169–179. - PubMed
-
- Anachkova, B. , Russev, G. , Poirier, G.G. , 1989. DNA replication and poly(ADP-ribosyl)ation of chromatin. Cytobios. 58, 19–28. - PubMed
-
- Arnaudeau, C. , Lundin, C. , Helleday, T. , 2001. DNA double-strand breaks associated with replication forks are predominantly repaired by homologous recombination involving an exchange mechanism in mammalian cells. J. Mol. Biol.. 307, 1235–1245. - PubMed
-
- Augustin, A. , Spenlehauer, C. , Dumond, H. , Menissier-De Murcia, J. , Piel, M. , Schmit, A.C. , 2003. PARP-3 localizes preferentially to the daughter centriole and interferes with the G1/S cell cycle progression. J. Cell Sci.. 116, 1551–1562. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

