Bcr-abl signals to desensitize chronic myeloid leukemia cells to IFNα via accelerating the degradation of its receptor
- PMID: 21821707
- PMCID: PMC3204736
- DOI: 10.1182/blood-2010-12-325373
Bcr-abl signals to desensitize chronic myeloid leukemia cells to IFNα via accelerating the degradation of its receptor
Abstract
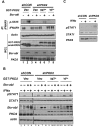
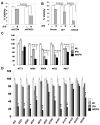
Constitutive activity of Bcr-abl fusion protein kinase causes chronic myeloid leukemia (CML). Inhibitors of Bcr-abl such as imatinib mesylate have replaced the cytokine IFNα as the primary treatment for the management of patients with this malignancy. We found that pretreatment of CML cells with imatinib mesylate augments the antigrowth effects of IFNα. Furthermore, introduction of Bcr-abl into non-CML cells inhibits the cellular responses to IFNα. This inhibition is mediated via a mechanism that involves activation of protein kinase D2. The latter promotes an accelerated phosphorylation-dependent degradation of the interferon-α/β receptor 1 chain of the type I interferon receptor, leading to attenuation of IFNα signaling. We discuss the relationship between Bcr-abl activity and IFNα signaling as a molecular basis of the combination of inhibitors of Bcr-abl and IFNα for CML treatment.
Figures
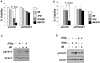



Comment in
-
Imatinib is receptive to a collaboration.Blood. 2011 Oct 13;118(15):4009-10. doi: 10.1182/blood-2011-08-373290. Blood. 2011. PMID: 21998331 No abstract available.
References
-
- Kharas MG, Fruman DA. ABL oncogenes and phosphoinositide 3-kinase: mechanism of activation and downstream effectors. Cancer Res. 2005;65(6):2047–2053. - PubMed
-
- Ren R. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer. 2005;5(3):172–183. - PubMed
-
- Branford S, Rudzki Z, Harper A, et al. Imatinib produces significantly superior molecular responses compared to interferon alfa plus cytarabine in patients with newly diagnosed chronic myeloid leukemia in chronic phase. Leukemia. 2003;17(12):2401–2409. - PubMed
-
- O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994–1004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

