Processing is required for a fully functional protein P30 in Mycoplasma pneumoniae gliding and cytadherence
- PMID: 21821772
- PMCID: PMC3187232
- DOI: 10.1128/JB.00104-11
Processing is required for a fully functional protein P30 in Mycoplasma pneumoniae gliding and cytadherence
Abstract
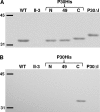
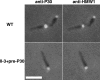
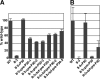
The cell wall-less prokaryote Mycoplasma pneumoniae causes bronchitis and atypical pneumonia in humans. Mycoplasma attachment to the host respiratory epithelium is required for colonization and mediated largely by a differentiated terminal organelle. P30 is an integral membrane protein located at the distal end of the terminal organelle. The P30 null mutant II-3 is unable to attach to host cells and nonmotile and has a branched cellular morphology compared to the wild type, indicating an important role for P30 in M. pneumoniae biology. P30 is predicted to have an N-terminal signal sequence, but the presence of such a motif has not been confirmed experimentally. In the current study we analyzed P30 derivatives having epitope tags engineered at various locations to demonstrate that posttranslational processing occurred in P30. Several potential cleavage sites predicted in silico were examined, and a processing-defective mutant was created to explore P30 maturation further. Our results suggested that signal peptide cleavage occurs between residues 52 and 53 to yield mature P30. The processing-defective mutant exhibited reduced gliding velocity and cytadherence, indicating that processing is required for fully functional maturation of P30. We speculate that P30 processing may trigger a conformational change in the extracellular domain or expose a binding site on the cytoplasmic domain to allow interaction with a binding partner as a part of functional maturation.
Figures


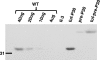
References
-
- Baseman J. B., et al. 1987. Identification of a 32-kilodalton protein of Mycoplasma pneumoniae associated with hemadsorption. Isr. J. Med. Sci. 23:474–479 - PubMed
-
- Biscardi S., et al. 2004. Mycoplasma pneumoniae and asthma in children. Clin. Infect. Dis. 38:1341–1346 - PubMed
-
- Collier A. M., Clyde W. A., Jr., Denny F. W. 1971. Mycoplasma pneumoniae in hamster tracheal organ culture: immunofluorescent and electron microscopic studies. Proc. Soc. Exp. Biol. Med. 136:569–573 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

