Tolerance induced by apoptotic antigen-coupled leukocytes is induced by PD-L1+ and IL-10-producing splenic macrophages and maintained by T regulatory cells
- PMID: 21821796
- PMCID: PMC3159828
- DOI: 10.4049/jimmunol.1004175
Tolerance induced by apoptotic antigen-coupled leukocytes is induced by PD-L1+ and IL-10-producing splenic macrophages and maintained by T regulatory cells
Abstract
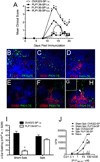
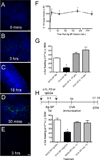
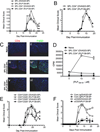
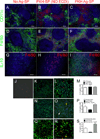
Ag-specific tolerance is a highly desired therapy for immune-mediated diseases. Intravenous infusion of protein/peptide Ags linked to syngeneic splenic leukocytes with ethylene carbodiimide (Ag-coupled splenocytes [Ag-SP]) has been demonstrated to be a highly efficient method for inducing peripheral, Ag-specific T cell tolerance for treatment of autoimmune disease. However, little is understood about the mechanisms underlying this therapy. In this study, we show that apoptotic Ag-SP accumulate in the splenic marginal zone, where their uptake by F4/80(+) macrophages induces production of IL-10, which upregulates the expression of the immunomodulatory costimulatory molecule PD-L1 that is essential for Ag-SP tolerance induction. Ag-SP infusion also induces T regulatory cells that are dispensable for tolerance induction but required for long-term tolerance maintenance. Collectively, these results indicate that Ag-SP tolerance recapitulates how tolerance is normally maintained in the hematopoietic compartment and highlight the interplay between the innate and adaptive immune systems in the induction of Ag-SP tolerance. To our knowledge, we show for the first time that tolerance results from the synergistic effects of two distinct mechanisms, PD-L1-dependent T cell-intrinsic unresponsiveness and the activation of T regulatory cells. These findings are particularly relevant as this tolerance protocol is currently being tested in a Phase I/IIa clinical trial in new-onset relapsing-remitting multiple sclerosis.
Figures



References
-
- Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat. Rev. Immunol. 2007;7:622–632. - PubMed
-
- Kohm AP, Turley DM, Miller SD. Targeting the TCR: T-cell receptor and peptide-specific tolerance-based strategies for restoring self-tolerance in CNS autoimmune disease. Int. Rev. Immunol. 2005;24:361–392. - PubMed
-
- Miller SD, Turley DM, Podojil JR. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat. Rev. Immunol. 2007;7:665–677. - PubMed
-
- Luo X, Pothoven KL, McCarthy D, DeGutes M, Martin A, Getts DR, Xia G, He J, Zhang X, Kaufman DB, Miller SD. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proc. Natl. Acad. Sci. USA. 2008;105:14527–14532. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

