Seeds of Brassicaceae weeds have an inherent or inducible response to the germination stimulant karrikinolide
- PMID: 21821831
- PMCID: PMC3177676
- DOI: 10.1093/aob/mcr198
Seeds of Brassicaceae weeds have an inherent or inducible response to the germination stimulant karrikinolide
Abstract
Background and aims: Karrikinolide (KAR(1)) is a smoke-derived chemical that can trigger seeds to germinate. A potential application for KAR(1) is for synchronizing the germination of weed seeds, thereby enhancing the efficiency of weed control efforts. Yet not all species germinate readily with KAR(1), and it is not known whether seemingly non-responsive species can be induced to respond. Here a major agronomic weed family, the Brassicaceae, is used to test the hypothesis that a stimulatory response to KAR(1) may be present in physiologically dormant seeds but may not be expressed under all circumstances.
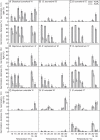
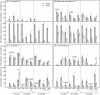
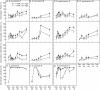
Methods: Seeds of eight Brassicaceae weed species (Brassica tournefortii, Raphanus raphanistrum, Sisymbrium orientale, S. erysimoides, Rapistrum rugosum, Lepidium africanum, Heliophila pusilla and Carrichtera annua) were tested for their response to 1 µm KAR(1) when freshly collected and following simulated and natural dormancy alleviation, which included wet-dry cycling, dry after-ripening, cold and warm stratification and a 2 year seed burial trial.
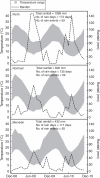
Key results: Seven of the eight Brassicaceae species tested were stimulated to germinate with KAR(1) when the seeds were fresh, and the remaining species became responsive to KAR(1) following wet-dry cycling and dry after-ripening. Light influenced the germination response of seeds to KAR(1), with the majority of species germinating better in darkness. Germination with and without KAR(1) fluctuated seasonally throughout the seed burial trial.
Conclusions: KAR(1) responses are more complex than simply stating whether a species is responsive or non-responsive; light and temperature conditions, dormancy state and seed lot all influence the sensitivity of seeds to KAR(1), and a response to KAR(1) can be induced. Three response types for generalizing KAR(1) responses are proposed, namely inherent, inducible and undetected. Given that responses to KAR(1) were either inherent or inducible in all 15 seed lots included in this study, the Brassicaceae may be an ideal target for future application of KAR(1) in weed management.
Figures



Similar articles
-
Prior hydration of Brassica tournefortii seeds reduces the stimulatory effect of karrikinolide on germination and increases seed sensitivity to abscisic acid.Ann Bot. 2010 Jun;105(6):1063-70. doi: 10.1093/aob/mcq061. Epub 2010 Mar 25. Ann Bot. 2010. PMID: 20348089 Free PMC article.
-
Comparison of germination responses of Anigozanthos flavidus (Haemodoraceae), Gyrostemon racemiger and Gyrostemon ramulosus (Gyrostemonaceae) to smoke-water and the smoke-derived compounds karrikinolide (KAR1) and glyceronitrile.Ann Bot. 2013 Mar;111(3):489-97. doi: 10.1093/aob/mcs300. Epub 2013 Jan 8. Ann Bot. 2013. PMID: 23299994 Free PMC article.
-
Parental environment changes the dormancy state and karrikinolide response of Brassica tournefortii seeds.Ann Bot. 2012 Jun;109(7):1369-78. doi: 10.1093/aob/mcs067. Epub 2012 Apr 3. Ann Bot. 2012. PMID: 22492259 Free PMC article.
-
Plant-Derived Smoke Affects Biochemical Mechanism on Plant Growth and Seed Germination.Int J Mol Sci. 2020 Oct 20;21(20):7760. doi: 10.3390/ijms21207760. Int J Mol Sci. 2020. PMID: 33092218 Free PMC article. Review.
-
Cover crops, hormones and herbicides: Priming an integrated weed management strategy.Plant Sci. 2020 Dec;301:110550. doi: 10.1016/j.plantsci.2020.110550. Epub 2020 Jun 20. Plant Sci. 2020. PMID: 33218616 Review.
Cited by
-
Regulation of Seed Dormancy and Germination Mechanisms in a Changing Environment.Int J Mol Sci. 2021 Jan 29;22(3):1357. doi: 10.3390/ijms22031357. Int J Mol Sci. 2021. PMID: 33572974 Free PMC article. Review.
-
Emerging Challenges and Opportunities for Education and Research in Weed Science.Front Plant Sci. 2017 Sep 5;8:1537. doi: 10.3389/fpls.2017.01537. eCollection 2017. Front Plant Sci. 2017. PMID: 28928765 Free PMC article. Review.
-
HTL/KAI2 signaling substitutes for light to control plant germination.PLoS Genet. 2024 Oct 21;20(10):e1011447. doi: 10.1371/journal.pgen.1011447. eCollection 2024 Oct. PLoS Genet. 2024. PMID: 39432524 Free PMC article.
-
Ecological Niche Shifts Affect the Potential Invasive Risk of Rapistrum rugosum (L.) All. in China.Front Plant Sci. 2022 Apr 15;13:827497. doi: 10.3389/fpls.2022.827497. eCollection 2022. Front Plant Sci. 2022. PMID: 35498683 Free PMC article.
-
An Updated Overview on the Regulation of Seed Germination.Plants (Basel). 2020 Jun 1;9(6):703. doi: 10.3390/plants9060703. Plants (Basel). 2020. PMID: 32492790 Free PMC article. Review.
References
-
- Adkins SW, Peters NCB. Smoke derived from burnt vegetation stimulates germination of arable weeds. Seed Science Research. 2001;11:213–222.
-
- Australian Government Bureau of Meteorology. Climate data online. 2010 http://www.bom.gov.au/climate/data . Accessed 4 Dec, 2010.
-
- Baskin CC, Baskin JM. Seeds: ecology, biogeogrphy, and evolution of dormancy and germination. San Diego: Academic Press; 2001.
-
- Baskin CC, Baskin JM. The natural history of soil seed banks of arable land. Weed Science. 2006;54:549–557.

