Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice
- PMID: 21821911
- PMCID: PMC3163958
- DOI: 10.1172/JCI46274
Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice
Abstract
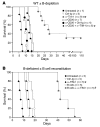
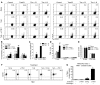
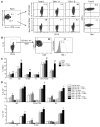
T cell Ig domain and mucin domain protein 1 (TIM-1) is a costimulatory molecule that regulates immune responses by modulating CD4+ T cell effector differentiation. However, the function of TIM-1 on other immune cell populations is unknown. Here, we show that in vivo in mice, TIM-1 is predominantly expressed on B rather than T cells. Importantly, TIM-1 was expressed by a large majority of IL-10-expressing regulatory B cells in all major B cell subpopulations, including transitional, marginal zone, and follicular B cells, as well as the B cell population characterized as CD1d(hi)CD5+. A low-affinity TIM-1-specific antibody that normally promotes tolerance in mice, actually accelerated (T cell-mediated) immune responsiveness in the absence of B cells. TIM-1+ B cells were highly enriched for IL-4 and IL-10 expression, promoted Th2 responses, and could directly transfer allograft tolerance. Both cytokine expression and number of TIM-1+ regulatory B cells (Bregs) were induced by TIM-1-specific antibody, and this was dependent on IL-4 signaling. Thus, TIM-1 is an inclusive marker for IL-10+ Bregs that can be induced by TIM-1 ligation. These findings suggest that TIM-1 may be a novel therapeutic target for modulating the immune response and provide insight into the signals involved in the generation and induction of Bregs.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

