Preclinical assessment of CNS drug action using eye movements in mice
- PMID: 21821912
- PMCID: PMC3163951
- DOI: 10.1172/JCI45557
Preclinical assessment of CNS drug action using eye movements in mice
Abstract
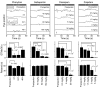
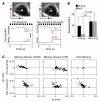
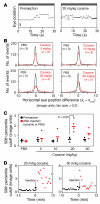
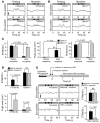
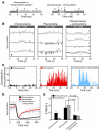
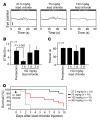
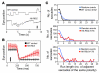
The drug development process for CNS indications is hampered by a paucity of preclinical tests that accurately predict drug efficacy in humans. Here, we show that a wide variety of CNS-active drugs induce characteristic alterations in visual stimulus-induced and/or spontaneous eye movements in mice. Active compounds included sedatives and antipsychotic, antidepressant, and antiseizure drugs as well as drugs of abuse, such as cocaine, morphine, and phencyclidine. The use of quantitative eye-movement analysis was demonstrated by comparing it with the commonly used rotarod test of motor coordination and by using eye movements to monitor pharmacokinetics, blood-brain barrier penetration, drug-receptor interactions, heavy metal toxicity, pharmacologic treatment in a model of schizophrenia, and degenerative CNS disease. We conclude that eye-movement analysis could complement existing animal tests to improve preclinical drug development.
Figures



Similar articles
-
Right frontal eye field has perceptual and oculomotor functions during optokinetic stimulation and nystagmus.J Neurophysiol. 2020 Feb 1;123(2):571-586. doi: 10.1152/jn.00468.2019. Epub 2019 Dec 25. J Neurophysiol. 2020. PMID: 31875488
-
Defective control and adaptation of reflex eye movements in mutant mice deficient in either the glutamate receptor delta2 subunit or Purkinje cells.Eur J Neurosci. 2005 Mar;21(5):1315-26. doi: 10.1111/j.1460-9568.2005.03946.x. Eur J Neurosci. 2005. PMID: 15813941
-
Alternating optokinetic nystagmus (OKN) induced by intermittent display of stationary gratings.Exp Brain Res. 2003 Feb;148(4):545-8. doi: 10.1007/s00221-002-1350-z. Epub 2003 Jan 15. Exp Brain Res. 2003. PMID: 12582841
-
Alteration of eye movements and motion perception in microgravity.Brain Res Brain Res Rev. 1998 Nov;28(1-2):161-72. doi: 10.1016/s0165-0173(98)00036-8. Brain Res Brain Res Rev. 1998. PMID: 9795198 Review.
-
Using eye movements to assess brain function in mice.Vision Res. 2004 Dec;44(28):3401-10. doi: 10.1016/j.visres.2004.09.011. Vision Res. 2004. PMID: 15536008 Review.
Cited by
-
A Systematic Review of the Protective Actions of Cat's Whiskers (Misai Kucing) on the Central Nervous System.Front Pharmacol. 2020 May 13;11:692. doi: 10.3389/fphar.2020.00692. eCollection 2020. Front Pharmacol. 2020. PMID: 32477146 Free PMC article.
-
Psychophysical testing in rodent models of glaucomatous optic neuropathy.Exp Eye Res. 2015 Dec;141:154-63. doi: 10.1016/j.exer.2015.06.025. Epub 2015 Jul 2. Exp Eye Res. 2015. PMID: 26144667 Free PMC article. Review.
-
Retracted: Allosteric Activators of Protein Phosphatase 2A Display Broad Antitumor Activity Mediated by Dephosphorylation of MYBL2.Cell. 2020 Apr 30;181(3):702-715.e20. doi: 10.1016/j.cell.2020.03.051. Epub 2020 Apr 20. Cell. 2020. Retraction in: Cell. 2022 Aug 4;185(16):3058. doi: 10.1016/j.cell.2022.07.008. PMID: 32315619 Free PMC article. Retracted.
-
Die Fledermaus: regarding optokinetic contrast sensitivity and light-adaptation, chicks are mice with wings.PLoS One. 2013 Sep 30;8(9):e75375. doi: 10.1371/journal.pone.0075375. eCollection 2013. PLoS One. 2013. PMID: 24098693 Free PMC article.
References
-
- Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3(8):711–715. - PubMed
-
- Pangalos MN, Schechter LE, Hurko O. Drug development for CNS disorders: strategies for balancing risk and reducing attrition. Nat Rev Drug Discov. 2007;6(7):521–532. - PubMed
-
- Reichel A. The role of blood-brain barrier studies in the pharmaceutical industry. Curr Drug Metab. 2006;7(2):183–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

