Characterization of the cell of origin for small cell lung cancer
- PMID: 21822053
- PMCID: PMC3219544
- DOI: 10.4161/cc.10.16.17012
Characterization of the cell of origin for small cell lung cancer
Abstract
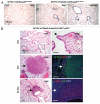
Small cell lung carcinoma (SCLC) is a neuroendocrine subtype of lung cancer that affects more than 200,000 people worldwide every year with a very high mortality rate. Here, we used a mouse genetics approach to characterize the cell of origin for SCLC; in this mouse model, tumors are initiated by the deletion of the Rb and p53 tumor suppressor genes in the lung epithelium of adult mice. We found that mouse SCLCs often arise in the lung epithelium, where neuroendocrine cells are located, and that the majority of early lesions were composed of proliferating neuroendocrine cells. In addition, mice in which Rb and p53 are deleted in a variety of non-neuroendocrine lung epithelial cells did not develop SCLC. These data indicate that SCLC likely arises from neuroendocrine cells in the lung.
Figures
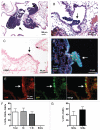
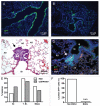
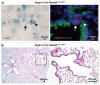
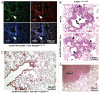
Comment in
-
Neuroendocrine cells: potential cells of origin for small cell lung carcinoma.Cell Cycle. 2011 Nov 1;10(21):3629-30. doi: 10.4161/cc.10.21.18034. Epub 2011 Nov 1. Cell Cycle. 2011. PMID: 22024916 Free PMC article. No abstract available.
-
Do neuroendocrine cells come up large in small cell lung cancer?Cell Cycle. 2011 Nov 1;10(21):3627. doi: 10.4161/cc.10.21.17688. Epub 2011 Nov 1. Cell Cycle. 2011. PMID: 22024918 No abstract available.
-
Small cell lung cancer: new insights into origins.Cell Cycle. 2011 Oct 15;10(20):3432. doi: 10.4161/cc.10.20.17664. Epub 2011 Oct 15. Cell Cycle. 2011. PMID: 22030465 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
