An autoinhibitory helix in the C-terminal region of phospholipase C-β mediates Gαq activation
- PMID: 21822282
- PMCID: PMC3168981
- DOI: 10.1038/nsmb.2095
An autoinhibitory helix in the C-terminal region of phospholipase C-β mediates Gαq activation
Abstract
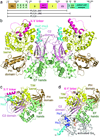
The enzyme phospholipase C-β (PLCβ) is a crucial regulator of intracellular calcium levels whose activity is controlled by heptahelical receptors that couple to members of the Gq family of heterotrimeric G proteins. We have determined atomic structures of two invertebrate homologs of PLCβ (PLC21) from cephalopod retina and identified a helix from the C-terminal regulatory region that interacts with a conserved surface of the catalytic core of the enzyme. Mutations designed to disrupt the analogous interaction in human PLCβ3 considerably increase basal activity and diminish stimulation by Gαq. Gαq binding requires displacement of the autoinhibitory helix from the catalytic core, thus providing an allosteric mechanism for activation of PLCβ.
Figures
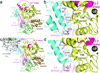

Similar articles
-
Activation of Phospholipase C β by Gβγ and Gαq Involves C-Terminal Rearrangement to Release Autoinhibition.Structure. 2020 Jul 7;28(7):810-819.e5. doi: 10.1016/j.str.2020.04.012. Epub 2020 May 12. Structure. 2020. PMID: 32402248 Free PMC article.
-
Gαq and the Phospholipase Cβ3 X-Y Linker Regulate Adsorption and Activity on Compressed Lipid Monolayers.Biochemistry. 2019 Aug 13;58(32):3454-3467. doi: 10.1021/acs.biochem.9b00441. Epub 2019 Jul 30. Biochemistry. 2019. PMID: 31322863 Free PMC article.
-
Full-length Gα(q)-phospholipase C-β3 structure reveals interfaces of the C-terminal coiled-coil domain.Nat Struct Mol Biol. 2013 Mar;20(3):355-62. doi: 10.1038/nsmb.2497. Epub 2013 Feb 3. Nat Struct Mol Biol. 2013. PMID: 23377541 Free PMC article.
-
Phospholipase Cβ connects G protein signaling with RNA interference.Adv Biol Regul. 2016 May;61:51-7. doi: 10.1016/j.jbior.2015.11.006. Epub 2015 Dec 2. Adv Biol Regul. 2016. PMID: 26746047 Free PMC article. Review.
-
Structural insights into phospholipase C-β function.Mol Pharmacol. 2013 Oct;84(4):488-500. doi: 10.1124/mol.113.087403. Epub 2013 Jul 23. Mol Pharmacol. 2013. PMID: 23880553 Free PMC article. Review.
Cited by
-
Structural and functional analysis of the regulator of G protein signaling 2-gαq complex.Structure. 2013 Mar 5;21(3):438-48. doi: 10.1016/j.str.2012.12.016. Epub 2013 Feb 21. Structure. 2013. PMID: 23434405 Free PMC article.
-
Molecular regulation of PLCβ signaling.Methods Enzymol. 2023;682:17-52. doi: 10.1016/bs.mie.2023.01.001. Epub 2023 Feb 22. Methods Enzymol. 2023. PMID: 36948701 Free PMC article. Review.
-
Hydrolysis rates of different small interfering RNAs (siRNAs) by the RNA silencing promoter complex, C3PO, determines their regulation by phospholipase Cβ.J Biol Chem. 2014 Feb 21;289(8):5134-44. doi: 10.1074/jbc.M113.531467. Epub 2013 Dec 12. J Biol Chem. 2014. PMID: 24338081 Free PMC article.
-
Gβγ activates PIP2 hydrolysis by recruiting and orienting PLCβ on the membrane surface.Proc Natl Acad Sci U S A. 2023 May 16;120(20):e2301121120. doi: 10.1073/pnas.2301121120. Epub 2023 May 12. Proc Natl Acad Sci U S A. 2023. PMID: 37172014 Free PMC article.
-
Characterization of the membrane interactions of phospholipase Cγ reveals key features of the active enzyme.Sci Adv. 2022 Jun 24;8(25):eabp9688. doi: 10.1126/sciadv.abp9688. Epub 2022 Jun 24. Sci Adv. 2022. PMID: 35749497 Free PMC article.
References
-
- Rebecchi MJ, Pentyala SN. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev. 2000;80:1291–1335. - PubMed
-
- Taylor SJ, Exton JH. Two a subunits of the Gq class of G proteins stimulate phosphoinositide phospholipase C-β1 activity. FEBS Lett. 1991;286:214–216. - PubMed
-
- Smrcka AV, Hepler JR, Brown KO, Sternweis PC. Regulation of polyphosphoinositide-specific phospholipase C activity by purified Gq. Science. 1991;251:804–807. - PubMed
-
- Park D, Jhon DY, Lee CW, Lee KH, Rhee SG. Activation of phospholipase C isozymes by G protein βγ subunits. J Biol Chem. 1993;268:4573–4576. - PubMed
METHODS ONLY REFERENCES
-
- Biddlecome GH, Berstein G, Ross EM. Regulation of phospholipase C-β1 by Gq and m1 muscarinic cholinergic receptor. Steady-state balance of receptor-mediated activation and GTPase-activating protein-promoted deactivation. J Biol Chem. 1996;271:7999–8007. - PubMed
-
- Tesmer VM, Kawano T, Shankaranarayanan A, Kozasa T, Tesmer JJ. Snapshot of activated G proteins at the membrane: the Gαq-GRK2-Gβγ complex. Science. 2005;310:1686–1690. - PubMed
-
- Mezzasalma TM, et al. Enhancing recombinant protein quality and yield by protein stability profiling. J Biomol Screen. 2007;12:418–428. - PubMed
-
- Ghosh M, Smrcka AV. Assay for G protein-dependent activation of phospholipase Cβ using purified protein components. Methods Mol Biol. 2004;237:67–75. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

