Oncogenic PIK3CA-driven mammary tumors frequently recur via PI3K pathway-dependent and PI3K pathway-independent mechanisms
- PMID: 21822287
- PMCID: PMC3169724
- DOI: 10.1038/nm.2402
Oncogenic PIK3CA-driven mammary tumors frequently recur via PI3K pathway-dependent and PI3K pathway-independent mechanisms
Abstract
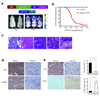
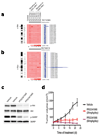
PIK3CA gain-of-function mutations are a common oncogenic event in human malignancy, making phosphatidylinositol 3-kinase (PI3K) a target for cancer therapy. Despite the promise of targeted therapy, resistance often develops, leading to treatment failure. To elucidate mechanisms of resistance to PI3K-targeted therapy, we constructed a mouse model of breast cancer conditionally expressing human PIK3CA(H1047R). Notably, most PIK3CA(H1047R)-driven mammary tumors recurred after PIK3CA(H1047R) inactivation. Genomic analyses of recurrent tumors revealed multiple lesions, including focal amplification of Met or Myc (also known as c-Met and c-Myc, respectively). Whereas Met amplification led to tumor survival dependent on activation of endogenous PI3K, tumors with Myc amplification became independent of the PI3K pathway. Functional analyses showed that Myc contributed to oncogene independence and resistance to PI3K inhibition. Notably, PIK3CA mutations and c-MYC elevation co-occur in a substantial fraction of human breast tumors. Together, these data suggest that c-MYC elevation represents a potential mechanism by which tumors develop resistance to current PI3K-targeted therapies.
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

