Autophagic deficiency is related to steroidogenic decline in aged rat Leydig cells
- PMID: 21822295
- PMCID: PMC3739565
- DOI: 10.1038/aja.2011.85
Autophagic deficiency is related to steroidogenic decline in aged rat Leydig cells
Abstract
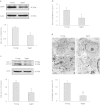
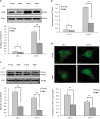
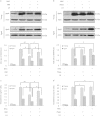
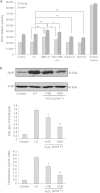
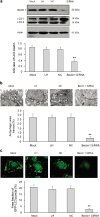
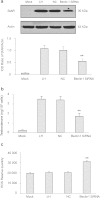
Late-onset hypogonadism (LOH) is closely related to secondary androgen deficiency in aged males, but the mechanism remains unclear. In this study, we found that reduced testosterone production in aged rat Leydig cells is associated with decreased autophagic activity. Primary rat Leydig cells and the TM3 mouse Leydig cell line were used to study the effect of autophagic deficiency on Leydig cell testosterone production. In Leydig cells from young and aged rats, treatment with wortmannin, an autophagy inhibitor, inhibited luteinising hormone (LH)-stimulated steroidogenic acute regulatory (StAR) protein expression and decreased testosterone production. In contrast, treatment with rapamycin, an autophagy activator, enhanced LH-stimulated steroidogenesis in Leydig cells from aged, but not young, rats. Intracellular reactive oxygen species (ROS) levels were increased in both young and aged Leydig cells treated with wortmannin but decreased only in aged Leydig cells treated with rapamycin. Furthermore, an increased level of ROS, induced by H(2)O(2), resulted in LH-stimulated steroidogenic inhibition. Finally, knockdown of Beclin 1 decreased LH-stimulated StAR expression and testosterone production in TM3 mouse Leydig cells, which were associated with increased intracellular ROS level. These results suggested that autophagic deficiency is related to steroidogenic decline in aged rat Leydig cells, which might be influenced by intracellular ROS levels.
Figures
Similar articles
-
[P70S6K is involved in the inhibition of testosterone production in TM3 mouse Leydig cells overexpressing Cox7a2].Zhonghua Nan Ke Xue. 2011 Apr;17(4):291-5. Zhonghua Nan Ke Xue. 2011. PMID: 21548202 Chinese.
-
Perfluorododecanoic acid-induced steroidogenic inhibition is associated with steroidogenic acute regulatory protein and reactive oxygen species in cAMP-stimulated Leydig cells.Toxicol Sci. 2010 Apr;114(2):285-94. doi: 10.1093/toxsci/kfq014. Epub 2010 Jan 25. Toxicol Sci. 2010. PMID: 20100736
-
Cox7a2 mediates steroidogenesis in TM3 mouse Leydig cells.Asian J Androl. 2006 Sep;8(5):589-94. doi: 10.1111/j.1745-7262.2006.00178.x. Epub 2006 Jun 5. Asian J Androl. 2006. PMID: 16752004
-
Leydig cell aging and hypogonadism.Exp Gerontol. 2015 Aug;68:87-91. doi: 10.1016/j.exger.2015.02.014. Epub 2015 Feb 18. Exp Gerontol. 2015. PMID: 25700847 Free PMC article. Review.
-
Regulation of Leydig cell steroidogenic function during aging.Biol Reprod. 2000 Oct;63(4):977-81. doi: 10.1095/biolreprod63.4.977. Biol Reprod. 2000. PMID: 10993816 Review.
Cited by
-
Nandrolone decanoate interferes with testosterone biosynthesis altering blood-testis barrier components.J Cell Mol Med. 2017 Aug;21(8):1636-1647. doi: 10.1111/jcmm.13092. Epub 2017 Feb 28. J Cell Mol Med. 2017. PMID: 28244681 Free PMC article.
-
Testosterone regulates the autophagic clearance of androgen binding protein in rat Sertoli cells.Sci Rep. 2015 Mar 9;5:8894. doi: 10.1038/srep08894. Sci Rep. 2015. PMID: 25745956 Free PMC article.
-
Current insights into the implications of m6A RNA methylation and autophagy interaction in human diseases.Cell Biosci. 2021 Jul 27;11(1):147. doi: 10.1186/s13578-021-00661-x. Cell Biosci. 2021. PMID: 34315538 Free PMC article. Review.
-
The Expanding Role of Mitochondria, Autophagy and Lipophagy in Steroidogenesis.Cells. 2021 Jul 22;10(8):1851. doi: 10.3390/cells10081851. Cells. 2021. PMID: 34440620 Free PMC article. Review.
-
Beclin-1 deficiency in the murine ovary results in the reduction of progesterone production to promote preterm labor.Proc Natl Acad Sci U S A. 2014 Oct 7;111(40):E4194-203. doi: 10.1073/pnas.1409323111. Epub 2014 Sep 22. Proc Natl Acad Sci U S A. 2014. PMID: 25246579 Free PMC article.
References
-
- Nieschlag E, Swerdloff R, Behre HM, Gooren LJ, Kaufman JM, et al. Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, and EAU recommendations. J Androl. 2006;27:135–7. - PubMed
-
- Jones TH. Late onset hypogonadism. BMJ. 2009;338:b352. - PubMed
-
- Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123–35. - PubMed
-
- Bassil N, Morley JE. Late-life onset hypogonadism: a review. Clin Geriatr Med. 2010;26:197–222. - PubMed
-
- Amano T, Imao T, Takemae K, Iwamoto T, Nakanome M. Testosterone replacement therapy by testosterone ointment relieves lower urinary tract symptoms in late onset hypogonadism patients. Aging Male. 2010;13:242–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

