Energetic manipulation of chloroplast protein import and the use of chemical cross-linkers to map protein-protein interactions
- PMID: 21822846
- PMCID: PMC4049570
- DOI: 10.1007/978-1-61779-234-2_18
Energetic manipulation of chloroplast protein import and the use of chemical cross-linkers to map protein-protein interactions
Abstract
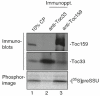
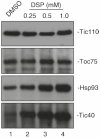
Most chloroplast proteins are synthesized in the cytosol as preproteins with N-terminal cleavable transit peptides and are imported into the organelle through the TOC-TIC translocon system. Import involves a complex set of recognition and membrane translocation steps that ensure the fidelity and unidirectional transport of the polypeptide across the double-membrane chloroplast envelope. To understand the mechanism of import, the molecular interactions and energetics of each step must be defined. Here, we describe the methods for capturing intermediates in the import process through the manipulation of the energy state of chloroplasts, and the use of two different chemical cross-linking approaches to examine the molecular interactions that mediate the import process and to assess the assembly state of the translocons. These approaches can be employed to identify sequential protein-protein interactions, and thereby dissect the pathway and roles of import components during protein import into chloroplasts.
Figures
Similar articles
-
Molecular Topology of the Transit Peptide during Chloroplast Protein Import.Plant Cell. 2018 Aug;30(8):1789-1806. doi: 10.1105/tpc.18.00172. Epub 2018 Jul 10. Plant Cell. 2018. PMID: 29991536 Free PMC article.
-
Prolines in Transit Peptides Are Crucial for Efficient Preprotein Translocation into Chloroplasts.Plant Physiol. 2018 Jan;176(1):663-677. doi: 10.1104/pp.17.01553. Epub 2017 Nov 20. Plant Physiol. 2018. PMID: 29158328 Free PMC article.
-
Protein import into chloroplasts via the Tic40-dependent and -independent pathways depends on the amino acid composition of the transit peptide.Biochem Biophys Res Commun. 2019 Oct 8;518(1):66-71. doi: 10.1016/j.bbrc.2019.08.009. Epub 2019 Aug 8. Biochem Biophys Res Commun. 2019. PMID: 31400859
-
The TOC GTPase Receptors: Regulators of the Fidelity, Specificity and Substrate Profiles of the General Protein Import Machinery of Chloroplasts.Protein J. 2019 Jun;38(3):343-350. doi: 10.1007/s10930-019-09846-3. Protein J. 2019. PMID: 31201619 Free PMC article. Review.
-
New insights into the mechanism of chloroplast protein import and its integration with protein quality control, organelle biogenesis and development.J Mol Biol. 2015 Mar 13;427(5):1038-1060. doi: 10.1016/j.jmb.2014.08.016. Epub 2014 Aug 28. J Mol Biol. 2015. PMID: 25174336 Free PMC article. Review.
Cited by
-
Arabidopsis ACYL CARRIER PROTEIN4 and RHOMBOID LIKE10 act independently in chloroplast phosphatidate synthesis.Plant Physiol. 2023 Nov 22;193(4):2661-2676. doi: 10.1093/plphys/kiad483. Plant Physiol. 2023. PMID: 37658850 Free PMC article.
-
Molecular Topology of the Transit Peptide during Chloroplast Protein Import.Plant Cell. 2018 Aug;30(8):1789-1806. doi: 10.1105/tpc.18.00172. Epub 2018 Jul 10. Plant Cell. 2018. PMID: 29991536 Free PMC article.
References
-
- Jarvis P. Organellar proteomics: chloroplasts in the spotlight. Curr. Biol. 2004;14:R317–R319. - PubMed
-
- Jarvis P. Targeting of nucleus-encoded proteins to chloroplasts in plants. New Phytol. 2008;179:257–285. - PubMed
-
- Inaba T, Schnell DJ. Protein trafficking to plastids: one theme, many variations. Biochem. J. 2008;413:15–28. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

