Clinicopathological correlations in corticobasal degeneration
- PMID: 21823158
- PMCID: PMC3154081
- DOI: 10.1002/ana.22424
Clinicopathological correlations in corticobasal degeneration
Abstract
Objective: To characterize cognitive and behavioral features, physical findings, and brain atrophy patterns in pathology-proven corticobasal degeneration (CBD) and corticobasal syndrome (CBS) with known histopathology.
Methods: We reviewed clinical and magnetic resonance imaging data in all patients evaluated at our center with either an autopsy diagnosis of CBD (n = 18) or clinical CBS at first presentation with known histopathology (n = 40). Atrophy patterns were compared using voxel-based morphometry.
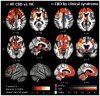
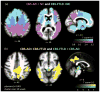
Results: CBD was associated with 4 clinical syndromes: progressive nonfluent aphasia (n = 5), behavioral variant frontotemporal dementia (n = 5), executive-motor (n = 7), and posterior cortical atrophy (n = 1). Behavioral or cognitive problems were the initial symptoms in 15 of 18 patients; less than half exhibited early motor findings. Compared to controls, CBD patients showed atrophy in dorsal prefrontal and perirolandic cortex, striatum, and brainstem (p < 0.001 uncorrected). The most common pathologic substrates for clinical CBS were CBD (35%), Alzheimer disease (AD, 23%), progressive supranuclear palsy (13%), and frontotemporal lobar degeneration (FTLD) with TDP inclusions (13%). CBS was associated with perirolandic atrophy irrespective of underlying pathology. In CBS due to FTLD (tau or TDP), atrophy extended into prefrontal cortex, striatum, and brainstem, whereas in CBS due to AD, atrophy extended into temporoparietal cortex and precuneus (p < 0.001 uncorrected).
Interpretation: Frontal lobe involvement is characteristic of CBD, and in many patients frontal, not parietal or basal ganglia, symptoms dominate early stage disease. CBS is driven by medial perirolandic dysfunction, but this anatomy is not specific to a single underlying histopathology. Antemortem prediction of CBD will remain challenging until clinical features of CBD are redefined, and sensitive, specific biomarkers are identified.
Copyright © 2011 American Neurological Association.
Figures


References
-
- Shelley BP, Hodges JR, Kipps CM, et al. Is the pathology of corticobasal syndrome predictable in life? Mov Disord. 2009;24:1593–1599. - PubMed
-
- Josephs KA, Petersen RC, Knopman DS, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006;66:41–48. - PubMed
-
- Boeve BF, Maraganore DM, Parisi JE, et al. Pathologic heterogeneity in clinically diagnosed corticobasal degeneration. Neurology. 1999;53:795–800. - PubMed
-
- Litvan I, Agid Y, Goetz C, et al. Accuracy of the clinical diagnosis of corticobasal degeneration: a clinicopathologic study. Neurology. 1997;48:119–125. - PubMed
-
- Josephs KA, Dickson DW. Diagnostic accuracy of progressive supranuclear palsy in the Society for Progressive Supranuclear Palsy brain bank. Mov Disord. 2003;18:1018–1026. - PubMed
Publication types
MeSH terms
Grants and funding
- AG-17586/AG/NIA NIH HHS/United States
- R01 AG031278/AG/NIA NIH HHS/United States
- AG10124/AG/NIA NIH HHS/United States
- P01 AG017586/AG/NIA NIH HHS/United States
- P50AG023501/AG/NIA NIH HHS/United States
- R01 AG038791/AG/NIA NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- T32AG23481/AG/NIA NIH HHS/United States
- T32 AG023481/AG/NIA NIH HHS/United States
- P01 AG019724/AG/NIA NIH HHS/United States
- K26 RR024858/RR/NCRR NIH HHS/United States
- R01AG038791/AG/NIA NIH HHS/United States
- AG023501/AG/NIA NIH HHS/United States
- R01AG031189/AG/NIA NIH HHS/United States
- K23AG021989/AG/NIA NIH HHS/United States
- AG19724/AG/NIA NIH HHS/United States
- P01AG019724/AG/NIA NIH HHS/United States
- P41RR023953/RR/NCRR NIH HHS/United States
- K26RR024858/RR/NCRR NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- P50 AG023501/AG/NIA NIH HHS/United States
- R03DC010878/DC/NIDCD NIH HHS/United States
- K23AG031861/AG/NIA NIH HHS/United States
- R01 AG031189/AG/NIA NIH HHS/United States
- K26 OD010927/OD/NIH HHS/United States
- K23 AG021989/AG/NIA NIH HHS/United States
- P41 RR023953/RR/NCRR NIH HHS/United States
- R01NS050915/NS/NINDS NIH HHS/United States
- R03 DC010878/DC/NIDCD NIH HHS/United States
- K23 AG031861/AG/NIA NIH HHS/United States
- R01 NS050915/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

