Discovery of a cytokinin deaminase
- PMID: 21823622
- PMCID: PMC3199332
- DOI: 10.1021/cb200198c
Discovery of a cytokinin deaminase
Abstract
An enzyme of unknown function within the amidohydrolase superfamily was discovered to catalyze the hydrolysis of N-6-substituted adenine derivatives, several of which are cytokinins. Cytokinins are a common type of plant hormone and N-6-substituted adenines are also found as modifications to tRNA. Patl2390, from Pseudoalteromonas atlantica T6c, was shown to hydrolytically deaminate N-6-isopentenyladenine to hypoxanthine and isopentenylamine with a k(cat)/K(m) of 1.2 × 10(7) M(-1) s(-1). Additional substrates include N-6-benzyl adenine, cis- and trans-zeatin, kinetin, O-6-methylguanine, N-6-butyladenine, N-6-methyladenine, N,N-dimethyladenine, 6-methoxypurine, 6-chloropurine, and 6-thiomethylpurine. This enzyme does not catalyze the deamination of adenine or adenosine. A comparative model of Patl2390 was computed using the three-dimensional crystal structure of Pa0148 (PDB code 3PAO ) as a structural template, and docking was used to refine the model to accommodate experimentally identified substrates. This is the first identification of an enzyme that will hydrolyze an N-6-substituted side chain larger than methylamine from adenine.
Figures
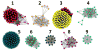


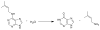
References
-
- Seibert CM, Raushel FM. Structural and catalytic diversity within the amidohydrolase superfamily. Biochemistry. 2005;44:6383–6391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

