Utilizing cell-matrix interactions to modulate gene transfer to stem cells inside hyaluronic acid hydrogels
- PMID: 21823632
- PMCID: PMC4104282
- DOI: 10.1021/mp200171d
Utilizing cell-matrix interactions to modulate gene transfer to stem cells inside hyaluronic acid hydrogels
Abstract
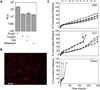
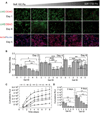
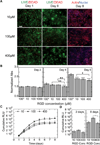
The effective delivery of DNA locally would increase the applicability of gene therapy in tissue regeneration, where diseased tissue is to be repaired in situ. One promising approach is to use hydrogel scaffolds to encapsulate and deliver plasmid DNA in the form of nanoparticles to the diseased tissue, so that cells infiltrating the scaffold are transfected to induce regeneration. This study focuses on the design of a DNA nanoparticle-loaded hydrogel scaffold. In particular, this study focuses on understanding how cell-matrix interactions affect gene transfer to adult stem cells cultured inside matrix metalloproteinase (MMP) degradable hyaluronic acid (HA) hydrogel scaffolds. HA was cross-linked to form a hydrogel material using a MMP degradable peptide and Michael addition chemistry. Gene transfer inside these hydrogel materials was assessed as a function of polyplex nitrogen to phosphate ratio (N/P = 5 to 12), matrix stiffness (100-1700 Pa), RGD (Arg-Gly-Asp) concentration (10-400 μM), and RGD presentation (0.2-4.7 RGDs per HA molecule). All variables were found to affect gene transfer to mouse mensenchymal stem cells culture inside the DNA loaded hydrogels. As expected, higher N/P ratios lead to higher gene transfer efficiency but also higher toxicity; softer hydrogels resulted in higher transgene expression than stiffer hydrogels, and an intermediate RGD concentration and RGD clustering resulted in higher transgene expression. We believe that the knowledge gained through this in vitro model can be utilized to design better scaffold-mediated gene delivery for local gene therapy.
Figures





References
-
- Kleinman HK, Philp D, Hoffman MP. Role of the extracellular matrix in morphogenesis. Curr Opin Biotechnol. 2003;14(5):526–532. - PubMed
-
- Bottaro DP, Liebmann-Vinson A, Heidaran MA. Molecular signaling in bioengineered tissue microenvironments. Ann N Y Acad Sci. 2002;961:143–153. - PubMed
-
- Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242(1):27–33. - PubMed
-
- Leach JB, Bivens KA, Patrick CW, Schmidt CE. Photocrosslinked hyaluronic acid hydrogels: Natural, biodegradable tissue engineering scaffolds. Biotechnology and Bioengineering. 2003;82(5):578–589. - PubMed
-
- Park YD, Tirelli N, Hubbell JA. Photopolymerized hyaluronic acid-based hydrogels and interpenetrating networks. Biomaterials. 2003;24(6):893–900. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

