A redesigned vancomycin engineered for dual D-Ala-D-ala And D-Ala-D-Lac binding exhibits potent antimicrobial activity against vancomycin-resistant bacteria
- PMID: 21823662
- PMCID: PMC3164945
- DOI: 10.1021/ja207142h
A redesigned vancomycin engineered for dual D-Ala-D-ala And D-Ala-D-Lac binding exhibits potent antimicrobial activity against vancomycin-resistant bacteria
Abstract
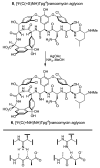
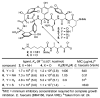
The emergence of bacteria resistant to vancomycin, often the antibiotic of last resort, poses a major health problem. Vancomycin-resistant bacteria sense a glycopeptide antibiotic challenge and remodel their cell wall precursor peptidoglycan terminus from d-Ala-d-Ala to d-Ala-d-Lac, reducing the binding of vancomycin to its target 1000-fold and accounting for the loss in antimicrobial activity. Here, we report [Ψ[C(═NH)NH]Tpg(4)]vancomycin aglycon designed to exhibit the dual binding to d-Ala-d-Ala and d-Ala-d-Lac needed to reinstate activity against vancomycin-resistant bacteria. Its binding to a model d-Ala-d-Ala ligand was found to be only 2-fold less than vancomycin aglycon and this affinity was maintained with a model d-Ala-d-Lac ligand, representing a 600-fold increase relative to vancomycin aglycon. Accurately reflecting these binding characteristics, it exhibits potent antimicrobial activity against vancomycin-resistant bacteria (MIC = 0.31 μg/mL, VanA VRE). Thus, a complementary single atom exchange in the vancomycin core structure (O → NH) to counter the single atom exchange in the cell wall precursors of resistant bacteria (NH → O) reinstates potent antimicrobial activity and charts a rational path forward for the development of antibiotics for the treatment of vancomycin-resistant bacterial infections.
Figures

References
-
- McCormick MH, Stark WM, Pittenger GE, Pittenger RC, McGuire JM. Antibiot. Annu. 1955-1956:606. - PubMed
-
- Nagarajan R, editor. Glycopeptide Antibiotics. Marcel Dekker; New York: 1994.
-
- Kahne D, Leimkuhler C, Lu W, Walsh CT. Chem. Rev. 2005;105:425. - PubMed
-
- Hubbard BK, Walsh CT. Angew. Chem., Int. Ed. 2003;42:730. - PubMed
-
- CDC National Nosocomial Infections Surveillance (NNIS) System Report, Data Summary from January 1992 Through June 2004, Issued October 2004. Am. J. Infect. Control 2004. 2003;32:470. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

