Recruitment of intracavernously injected adipose-derived stem cells to the major pelvic ganglion improves erectile function in a rat model of cavernous nerve injury
- PMID: 21824718
- PMCID: PMC3226917
- DOI: 10.1016/j.eururo.2011.07.061
Recruitment of intracavernously injected adipose-derived stem cells to the major pelvic ganglion improves erectile function in a rat model of cavernous nerve injury
Abstract
Background: Intracavernous (IC) injection of stem cells has been shown to ameliorate cavernous-nerve (CN) injury-induced erectile dysfunction (ED). However, the mechanisms of action of adipose-derived stem cells (ADSC) remain unclear.
Objectives: To investigate the mechanism of action and fate of IC injected ADSC in a rat model of CN crush injury.
Design, setting, and participants: Sprague-Dawley rats (n=110) were randomly divided into five groups. Thirty-five rats underwent sham surgery and IC injection of ADSC (n=25) or vehicle (n=10). Another 75 rats underwent bilateral CN crush injury and were treated with vehicle or ADSC injected either IC or in the dorsal penile perineural space. At 1, 3, 7 (n=5), and 28 d (n=10) postsurgery, penile tissues and major pelvic ganglia (MPG) were harvested for histology. ADSC were labeled with 5-ethynyl-2-deoxyuridine (EdU) before treatment. Rats in the 28-d groups were examined for erectile function prior to tissue harvest.
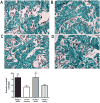
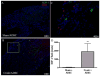
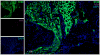
Measurements: IC pressure recording on CN electrostimulation, immunohistochemistry of the penis and the MPG, and number of EdU-positive (EdU+) cells in the injection site and the MPG.
Results and limitations: IC, but not perineural, injection of ADSC resulted in significantly improved erectile function. Significantly more EdU+ ADSC appeared in the MPG of animals with CN injury and IC injection of ADSC compared with those injected perineurally and those in the sham group. One day after crush injury, stromal cell-derived factor-1 (SDF-1) was upregulated in the MPG, providing an incentive for ADSC recruitment toward the MPG. Neuroregeneration was observed in the group that underwent IC injection of ADSC, and IC ADSC treatment had beneficial effects on the smooth muscle/collagen ratio in the corpus cavernosum.
Conclusions: CN injury upregulates SDF-1 expression in the MPG and thereby attracts intracavernously injected ADSC. At the MPG, ADSC exert neuroregenerative effects on the cell bodies of injured nerves, resulting in enhanced erectile response.
Copyright © 2011 European Association of Urology. Published by Elsevier B.V. All rights reserved.
Figures




References
-
- Heidenreich A, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59:61–71. - PubMed
-
- Dubbelman YD, Dohle GR, Schröder FH. Sexual function before and after radical retropubic prostatectomy: a systematic review of prognostic indicators for a successful outcome. Eur Urol. 2006;50:711–8. - PubMed
-
- Montorsi F, Brock G, Lee J, et al. Effect of nightly versus on-demand vardenafil on recovery of erectile function in men following bilateral nerve-sparing radical prostatectomy. Eur Urol. 2008;54:924–31. - PubMed
-
- Hatzimouratidis K, Burnett AL, Hatzichristou D, McCullough AR, Montorsi F, Mulhall JP. Phosphodiesterase type 5 inhibitors in postprostatectomy erectile dysfunction: a critical analysis of the basic science rationale and clinical application. Eur Urol. 2009;55:334–47. - PubMed
-
- Bochinski D, Lin GT, Nunes L, et al. The effect of neural embryonic stem cell therapy in a rat model of cavernosal nerve injury. BJU Int. 2004;94:904–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

