Striking In vivo phenotype of a disease-associated human SCN5A mutation producing minimal changes in vitro
- PMID: 21824921
- PMCID: PMC3297976
- DOI: 10.1161/CIRCULATIONAHA.110.987248
Striking In vivo phenotype of a disease-associated human SCN5A mutation producing minimal changes in vitro
Abstract
Background: The D1275N SCN5A mutation has been associated with a range of unusual phenotypes, including conduction disease and dilated cardiomyopathy, as well as atrial and ventricular tachyarrhythmias. However, when D1275N is studied in heterologous expression systems, most studies show near-normal sodium channel function. Thus, the relationship of the variant to the clinical phenotypes remains uncertain.
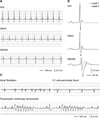
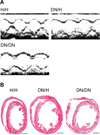
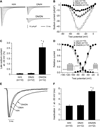
Methods and results: We identified D1275N in a patient with atrial flutter, atrial standstill, conduction disease, and sinus node dysfunction. There was no major difference in biophysical properties between wild-type and D1275N channels expressed in Chinese hamster ovary cells or tsA201 cells in the absence or presence of β1 subunits. To determine D1275N function in vivo, the Scn5a locus was modified to knock out the mouse gene, and the full-length wild-type (H) or D1275N (DN) human SCN5A cDNAs were then inserted at the modified locus by recombinase mediated cassette exchange. Mice carrying the DN allele displayed slow conduction, heart block, atrial fibrillation, ventricular tachycardia, and a dilated cardiomyopathy phenotype, with no significant fibrosis or myocyte disarray on histological examination. The DN allele conferred gene-dose-dependent increases in SCN5A mRNA abundance but reduced sodium channel protein abundance and peak sodium current amplitudes (H/H, 41.0±2.9 pA/pF at -30 mV; DN/H, 19.2±3.1 pA/pF, P<0.001 vs. H/H; DN/DN, 9.3±1.1 pA/pF, P<0.001 versus H/H).
Conclusions: Although D1275N produces near-normal currents in multiple heterologous expression experiments, our data establish this variant as a pathological mutation that generates conduction slowing, arrhythmias, and a dilated cardiomyopathy phenotype by reducing cardiac sodium current.
Figures





Comment in
-
Defining the disconnect between in vitro models and human arrhythmogenic disease: context matters.Circulation. 2011 Aug 30;124(9):993-5. doi: 10.1161/CIRCULATIONAHA.111.051466. Circulation. 2011. PMID: 21875919 Free PMC article. No abstract available.
References
-
- Wang Q, Shen J, Splawski I, Atkinson D, Li Z, Robinson JL, Moss AJ, Towbin JA, Keating MT. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80:805–811. - PubMed
-
- Chen Q, Kirsch GE, Zhang D, Brugada R, Brugada J, Brugada P, Potenza D, Moya A, Borggrefe M, Breithardt G, Ortiz-Lopez R, Wang Z, Antzelevitch C, O'Brien RE, Schulze-Bahr E, Keating MT, Towbin JA, Wang Q. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature. 1998;392:293–296. - PubMed
-
- Schott JJ, Alshinawi C, Kyndt F, Probst V, Hoorntje TM, Hulsbeek M, Wilde AA, Escande D, Mannens MM, Le Marec H. Cardiac conduction defects associate with mutations in SCN5A. Nat Genet. 1999;23:20–21. - PubMed
-
- Ellinor PT, Nam EG, Shea MA, Milan DJ, Ruskin JN, MacRae CA. Cardiac sodium channel mutation in atrial fibrillation. Heart Rhythm. 2008;5:99–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL049989/HL/NHLBI NIH HHS/United States
- DK58404/DK/NIDDK NIH HHS/United States
- U19 HL065962/HL/NHLBI NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- U01 HL065962/HL/NHLBI NIH HHS/United States
- U01 DK072473/DK/NIDDK NIH HHS/United States
- EY08126/EY/NEI NIH HHS/United States
- R01 HL088635/HL/NHLBI NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- DK59637/DK/NIDDK NIH HHS/United States
- U24 DK59637/DK/NIDDK NIH HHS/United States
- CA68485/CA/NCI NIH HHS/United States
- HD15052/HD/NICHD NIH HHS/United States
- P01 DK042502/DK/NIDDK NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- HL65962/HL/NHLBI NIH HHS/United States
- U19 DK042502/DK/NIDDK NIH HHS/United States
- P30 HD015052/HD/NICHD NIH HHS/United States
- HL49989/HL/NHLBI NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- DK72473/DK/NIDDK NIH HHS/United States
- DK42502/DK/NIDDK NIH HHS/United States
- DK20593/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

