The mitochondrial import protein Mim1 promotes biogenesis of multispanning outer membrane proteins
- PMID: 21825073
- PMCID: PMC3153637
- DOI: 10.1083/jcb.201102044
The mitochondrial import protein Mim1 promotes biogenesis of multispanning outer membrane proteins
Abstract
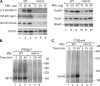
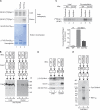
The mitochondrial outer membrane contains translocase complexes for the import of precursor proteins. The translocase of the outer membrane complex functions as a general preprotein entry gate, whereas the sorting and assembly machinery complex mediates membrane insertion of β-barrel proteins of the outer membrane. Several α-helical outer membrane proteins are known to carry multiple transmembrane segments; however, only limited information is available on the biogenesis of these proteins. We report that mitochondria lacking the mitochondrial import protein 1 (Mim1) are impaired in the biogenesis of multispanning outer membrane proteins, whereas overexpression of Mim1 stimulates their import. The Mim1 complex cooperates with the receptor Tom70 in binding of precursor proteins and promotes their insertion and assembly into the outer membrane. We conclude that the Mim1 complex plays a central role in the import of α-helical outer membrane proteins with multiple transmembrane segments.
Figures



References
-
- Ahting U., Waizenegger T., Neupert W., Rapaport D. 2005. Signal-anchored proteins follow a unique insertion pathway into the outer membrane of mitochondria. J. Biol. Chem. 280:48–53. - PubMed
-
- Becker T., Pfannschmidt S., Guiard B., Stojanovski D., Milenkovic D., Kutik S., Pfanner N., Meisinger C., Wiedemann N. 2008. Biogenesis of the mitochondrial TOM complex: Mim1 promotes insertion and assembly of signal-anchored receptors. J. Biol. Chem. 283:120–127. 10.1074/jbc.M706997200 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

