Marine microgels as a source of cloud condensation nuclei in the high Arctic
- PMID: 21825118
- PMCID: PMC3158224
- DOI: 10.1073/pnas.1102457108
Marine microgels as a source of cloud condensation nuclei in the high Arctic
Abstract
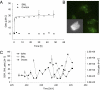
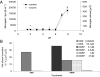
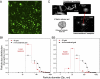
Marine microgels play an important role in regulating ocean basin-scale biogeochemical dynamics. In this paper, we demonstrate that, in the high Arctic, marine gels with unique physicochemical characteristics originate in the organic material produced by ice algae and/or phytoplankton in the surface water. The polymers in this dissolved organic pool assembled faster and with higher microgel yields than at other latitudes. The reversible phase transitions shown by these Arctic marine gels, as a function of pH, dimethylsulfide, and dimethylsulfoniopropionate concentrations, stimulate the gels to attain sizes below 1 μm in diameter. These marine gels were identified with an antibody probe specific toward material from the surface waters, sized, and quantified in airborne aerosol, fog, and cloud water, strongly suggesting that they dominate the available cloud condensation nuclei number population in the high Arctic (north of 80°N) during the summer season. Knowledge about emergent properties of marine gels provides important new insights into the processes controlling cloud formation and radiative forcing, and links the biology at the ocean surface with cloud properties and climate over the central Arctic Ocean and, probably, all oceans.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Solomon S, et al. Climate change 2007: The physical science basis, contribution of working group 1 to the fourth assessment report of the Intergovernmental Panel on Climate Change. In: Solomon S, et al., editors. Intergovernmental Panel on Climate Change. New York, NY: Cambridge University Press; 2007. p. 996.
-
- Koåhler H. The nucleus in and the growth of hygroscopic droplets. Trans Faraday Soc. 1936;32:1152–1161.
-
- Intrieri J, et al. An annual cycle of Arctic surface cloud forcing at SHEBA. J Geophys Res Oceans. 2002;107 10.1029/2000JC000439.
-
- Leck C, Persson C. Seasonal and short-term variability in dimethyl sulfide, sulfur dioxide and biogenic sulfur and sea salt aerosol particles in the arctic marine boundary layer during summer and autumn. Tellus. 1996;48B:272–299.
-
- Leck C, Bigg K. Biogenic particles in the surface microlayer and overlaying atmosphere in the central Arctic Ocean during summer. Tellus. 2005;57B:305–316.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

