Glycosylation of the enhanced aromatic sequon is similarly stabilizing in three distinct reverse turn contexts
- PMID: 21825145
- PMCID: PMC3161607
- DOI: 10.1073/pnas.1105880108
Glycosylation of the enhanced aromatic sequon is similarly stabilizing in three distinct reverse turn contexts
Abstract
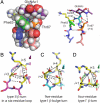
Cotranslational N-glycosylation can accelerate protein folding, slow protein unfolding, and increase protein stability, but the molecular basis for these energetic effects is incompletely understood. N-glycosylation of proteins at naïve sites could be a useful strategy for stabilizing proteins in therapeutic and research applications, but without engineering guidelines, often results in unpredictable changes to protein energetics. We recently introduced the enhanced aromatic sequon as a family of portable structural motifs that are stabilized upon glycosylation in specific reverse turn contexts: a five-residue type I β-turn harboring a G1 β-bulge (using a Phe-Yyy-Asn-Xxx-Thr sequon) and a type II β-turn within a six-residue loop (using a Phe-Yyy-Zzz-Asn-Xxx-Thr sequon) [Culyba EK, et al. (2011) Science 331:571-575]. Here we show that glycosylating a new enhanced aromatic sequon, Phe-Asn-Xxx-Thr, in a type I' β-turn stabilizes the Pin 1 WW domain. Comparing the energetic effects of glycosylating these three enhanced aromatic sequons in the same host WW domain revealed that the glycosylation-mediated stabilization is greatest for the enhanced aromatic sequon complementary to the type I β-turn with a G1 β-bulge. However, the portion of the stabilization from the tripartite interaction between Phe, Asn(GlcNAc), and Thr is similar for each enhanced aromatic sequon in its respective reverse turn context. Adding the Phe-Asn-Xxx-Thr motif (in a type I' β-turn) to the enhanced aromatic sequon family doubles the number of proteins that can be stabilized by glycosylation without having to alter the native reverse turn type.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
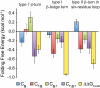
 , blue bars); (2) the two-way interaction between Phe16 and Asn(GlcNAc)19 (
, blue bars); (2) the two-way interaction between Phe16 and Asn(GlcNAc)19 ( , red bars); (3) the two-way interaction between Asn(GlcNAc)19 and Thr21 (
, red bars); (3) the two-way interaction between Asn(GlcNAc)19 and Thr21 ( , green bars); and (4) the three-way interaction between Phe16, Asn(GlcNAc)19, and Thr21 (
, green bars); and (4) the three-way interaction between Phe16, Asn(GlcNAc)19, and Thr21 ( , purple bars).
, purple bars).  ,
,  ,
,  , and
, and  , are parameters obtained from least-squares regression of Eq. 1; error bars represent the corresponding standard errors.
, are parameters obtained from least-squares regression of Eq. 1; error bars represent the corresponding standard errors.References
-
- Imperiali B. Protein glycosylation: The clash of the titans. Acc Chem Res. 1997;30:452–459.
-
- Yan A, Lennarz WJ. Unraveling the mechanism of protein N-glycosylation. J Biol Chem. 2005;280:3121–3124. - PubMed
-
- Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. - PubMed
-
- Kelleher DJ, Gilmore R. An evolving view of the eukaryotic oligosaccharyltransferase. Glycobiology. 2006;16:47R–62R. - PubMed
-
- Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291:2364–2369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

