A randomized, phase II, biomarker-selected study comparing erlotinib to erlotinib intercalated with chemotherapy in first-line therapy for advanced non-small-cell lung cancer
- PMID: 21825259
- PMCID: PMC3179254
- DOI: 10.1200/JCO.2010.34.4929
A randomized, phase II, biomarker-selected study comparing erlotinib to erlotinib intercalated with chemotherapy in first-line therapy for advanced non-small-cell lung cancer
Erratum in
- J Clin Oncol. 2011 Oct 10;29(29):3948. Camidge, Ross [corrected to Camidge, D Ross]
Abstract
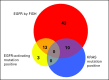
Purpose: Erlotinib prolongs survival in patients with advanced non-small-cell lung cancer (NSCLC). We report the results of a randomized, phase II study of erlotinib alone or intercalated with chemotherapy (CT + erlotinib) in chemotherapy-naïve patients with advanced NSCLC who were positive for epidermal growth factor receptor (EGFR) protein expression and/or with high EGFR gene copy number.
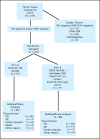
Patients and methods: A total of 143 patients were randomly assigned to either erlotinib 150 mg daily orally until disease progression (PD) occurred or to chemotherapy with paclitaxel 200 mg/m(2) intravenously (IV) and carboplatin dosed by creatinine clearance (AUC 6) IV on day 1 intercalated with erlotinib 150 mg orally on days 2 through 15 every 3 weeks for four cycles followed by erlotinib 150 mg orally until PD occurred (CT + erlotinib). The primary end point was 6-month progression-free survival (PFS); secondary end points included response rate, PFS, and survival. EGFR, KRAS mutation, EGFR fluorescent in situ hybridization and immunohistochemistry, and E-cadherin and vimentin protein levels were also assessed.
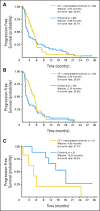
Results: Six-month PFS rates were 26% and 31% for the two arms (CT + erlotinib and erlotinib alone, respectively). Both were less than the historical control of 45% (P = .001 and P = .011, respectively). Median PFS times were 4.57 and 2.69 months, respectively. Patients with tumors harboring EGFR activating mutations fared better on erlotinib alone (median PFS, 18.2 months v 4.9 months for CT + erlotinib).
Conclusion: The feasibility of a multicenter biomarker-driven study was demonstrated, but neither treatment arms exceeded historical controls. This study does not support combined chemotherapy and erlotinib in first-line treatment of EGFR-selected advanced NSCLC, and the patients with tumors harboring EGFR mutations had a better outcome on erlotinib alone.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- Shepherd FA, Rodrigues PereiraJ, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. - PubMed
-
- Cappuzzo F, Ciuleanu T, Stelmakh L, et al. SATURN: A double-blind, randomized, phase III study of maintenance erlotinib versus placebo following nonprogression with first-line platinum-based chemotherapy in patients with advanced NSCLC. Lancet Oncol. 2010;11:521–529.
-
- Zhou C, Wu Y-L, Chen G, et al. Efficacy results from the randomized phase III optimal study comparing first-line erlotinib versus carboplatin plus gemcitabine in Chinese advanced non-small-cell lung cancer patients with EGFR activating mutations. Presented at the 35th ESMO Congress; October 8-12, 2010; Milan, Italy.
-
- Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non–small-cell lung cancer: The Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–1552. - PubMed
-
- Herbst RS, Prager D, Hermann R, et al. TRIBUTE: A phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non–small-cell lung cancer. J Clin Oncol. 2005;23:5892–5899. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

