Safety and efficacy of HIV hyperimmune globulin for prevention of mother-to-child HIV transmission in HIV-1-infected pregnant women and their infants in Kampala, Uganda (HIVIGLOB/NVP STUDY)
- PMID: 21826009
- PMCID: PMC3204156
- DOI: 10.1097/QAI.0b013e31822f8914
Safety and efficacy of HIV hyperimmune globulin for prevention of mother-to-child HIV transmission in HIV-1-infected pregnant women and their infants in Kampala, Uganda (HIVIGLOB/NVP STUDY)
Abstract
Background: This phase III, randomized, clinical trial compared single-dose nevirapine (sdNVP) plus HIV hyperimmune globulin (HIVIGLOB) with sdNVP alone for preventing maternal-to-child transmission of HIV. Primary objectives were to determine rates of HIV infection among infants and to assess the safety of HIVIGLOB in combination with sdNVP in HIV-infected Ugandan pregnant women and their infants.
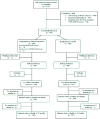
Methods: Mother-infant pairs were randomized to receive 200 mg of nevirapine to women in labor and 2 mg/kg NVP to newborns within 72 hours after birth (sdNVP arm) or to receive sdNVP plus a single intravenous 240-mL dose of HIVIGLOB given to women at 36- to 38-week gestation and a single intravenous 24-mL dose to newborns within 18 hours of birth (HIVIGLOB/sdNVP arm). Risk of HIV infection was determined using Kaplan-Meier and risk ratio estimates at birth, 2, 6, 14 weeks, 6, and 12 months of age.
Results: Intent-to-treat analysis included 198 HIVIGLOB/sdNVP and 294 sdNVP mother-infant pairs. At 6 months of age, the primary endpoint, there was no statistically significant difference in HIV transmission in the HIVIGLOB/sdNVP arm vs. the sdNVP arm [18.7% vs. 15.0%; risk ratio = 1.240 (95% confidence interval: 0.833 to 1.846); P = 0.290]. Similarly, the proportion of serious adverse events in the HIVIGLOB/sdNVP and sdNVP arms, respectively, for mothers (18.9% vs. 19.3%; P = 0.91) and infants (62.6% vs. 59.5%; P = 0.51) was not significantly different.
Conclusions: Giving mother-infant pairs an infusion of peripartum HIV hyperimmune globulin in addition to sdNVP for preventing maternal-to-child transmission was as safe as sdNVP alone but was no more effective than sdNVP alone in preventing HIV transmission.
Trial registration: ClinicalTrials.gov NCT00639938.
Conflict of interest statement
Figures
Comment in
-
Prevention of mother-to-child HIV-1 transmission--why we still need a preventive HIV immunization strategy.J Acquir Immune Defic Syndr. 2011 Dec 1;58(4):359-62. doi: 10.1097/QAI.0b013e318235517e. J Acquir Immune Defic Syndr. 2011. PMID: 21909031 No abstract available.
-
Were the interests of the vulnerable truly served? The predictable failure of HIVIG.J Acquir Immune Defic Syndr. 2012 Sep 1;61(1):e8-10; author reply e10-2. doi: 10.1097/QAI.0b013e318253a5dc. J Acquir Immune Defic Syndr. 2012. PMID: 22918129 No abstract available.
References
-
- UNAIDS. UNAIDS Report on the Global AIDS 2010
-
- De Cock KM, Fowler MG, Mercier E, et al. Prevention of mother-to-child HIV transmission in resource-poor countries: Translating research into policy and practice. JAMA. 2000;283:1175–1182. - PubMed
-
- Nduati R, John G, Mbori-Ngacha D, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: A randomized clinical trial. JAMA. 2000;283:1167–1174. - PubMed
-
- Moodley D, Moodley J, Coovadia H, et al. A multicenter randomized controlled trial of nevirapine versus a combination of zidovudine and lamivudine to reduce intrapartum and early postpartum mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis. 2003;187:725–735. - PubMed
-
- Miotti PG, Taha TE, Kumwenda NI, et al. HIV transmission through breastfeeding: A study in Malawi. JAMA. 1999;282:744–749. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical