Medical treatment of benign prostatic hyperplasia
- PMID: 21826125
- PMCID: PMC3151584
Medical treatment of benign prostatic hyperplasia
Abstract
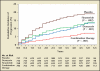
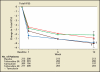
Medical therapy for the treatment of benign prostatic hyperplasia (BPH) became an accepted standard of care in the 1990s following the reports of randomized, double-blind, placebo-controlled studies showing that finasteride, a 5-α reductase inhibitor, and terazosin, an α-blocker, significantly improved lower urinary tract symptoms and increased peak urinary flow rates in men with BPH. This article reviews novel approaches to the pharmacological treatment of BPH.
Keywords: Benign prostatic hyperplasia; Combination therapy; Lower urinary tract symptoms; Monotherapy.
Figures





References
-
- Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474–479. - PubMed
-
- Shapiro E, Becich MJ, Hartanto V, Lepor H. The relative proportion of stromal and epithelial hyperplasia is related to the development of symptomatic benign prostate hyperplasia. J Urol. 1992;147:1293–1297. - PubMed
-
- Lepor H, Rigaud G. The efficacy of transurethral resection of the prostate in men with moderate symptoms of prostatism. J Urol. 1990;143:533–537. - PubMed
-
- The Finasteride Study Group, authors. Finasteride (MK-906) in the treatment of benign prostatic hyperplasia. Prostate. 1993;22:291–299. - PubMed
-
- Lepor H, Auerbach S, Puras-Baez A, et al. A randomized, placebo-controlled multicenter study of the efficacy and safety of terazosin in the treatment of benign prostatic hyperplasia. J Urol. 1992;148:1467–1474. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
