Synergistic Apoptosis-Inducing Antileukemic Effects of Arsenic Trioxide and Mucuna macrocarpa Stem Extract in Human Leukemic Cells via a Reactive Oxygen Species-Dependent Mechanism
- PMID: 21826188
- PMCID: PMC3150200
- DOI: 10.1155/2012/921430
Synergistic Apoptosis-Inducing Antileukemic Effects of Arsenic Trioxide and Mucuna macrocarpa Stem Extract in Human Leukemic Cells via a Reactive Oxygen Species-Dependent Mechanism
Abstract
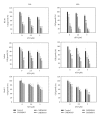
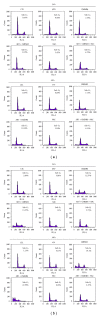
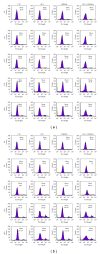
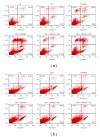
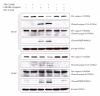
The objective of this study was to examine the potential of enhancing the antileukemic activity of arsenic trioxide (ATO) by combining it with a folk remedy, crude methanolic extract of Mucuna macrocarpa (CMEMM). Human leukemia cells HL-60, Jurkat, and Molt-3 were treated with various doses of ATO, CMEMM, and combinations thereof for 24 and 48 h. Results indicated that the combination of 2.5 μM ATO and 50 μg/mL CMEMM synergistically inhibited cell proliferation in HL-60 and Jurkat cell lines. Apoptosis triggered by ATO/CMEMM treatment was confirmed by accumulation of cells in the sub-G(1) phase in cell cycle analyses, characteristic apoptotic nuclear fragmentation, and increased percentage of annexin V-positive apoptotic cells. Such combination treatments also led to elevation of reactive oxygen species (ROS). The antioxidants N-acetyl cysteine (NAC), butylated hydroxytoluene, and α-tocopherol prevented cells from ATO/CMEMM-induced apoptosis. The ATO/CMEMM-induced activation of caspase-3 and caspase-9 can be blocked by NAC. In summary, these results suggest that ATO/CMEMM combination treatment exerts synergistic apoptosis-inducing effects in human leukemic cells through a ROS-dependent mechanism and may provide a promising antileukemic approach in the future.
Figures

Similar articles
-
In vitro and in vivo apoptosis-inducing antileukemic effects of Mucuna macrocarpa stem extract on HL-60 human leukemia cells.Integr Cancer Ther. 2010 Sep;9(3):298-308. doi: 10.1177/1534735410378661. Epub 2010 Aug 16. Integr Cancer Ther. 2010. PMID: 20713376
-
Genistein selectively potentiates arsenic trioxide-induced apoptosis in human leukemia cells via reactive oxygen species generation and activation of reactive oxygen species-inducible protein kinases (p38-MAPK, AMPK).Int J Cancer. 2008 Sep 1;123(5):1205-14. doi: 10.1002/ijc.23639. Int J Cancer. 2008. PMID: 18546268
-
The synergistic antitumor activity of arsenic trioxide and vitamin K2 in HL-60 cells involves increased ROS generation and regulation of the ROS-dependent MAPK signaling pathway.Pharmazie. 2013 Oct;68(10):839-45. Pharmazie. 2013. PMID: 24273890
-
Combination of Arsenic Trioxide and Valproic Acid Efficiently Inhibits Growth of Lung Cancer Cells via G2/M-Phase Arrest and Apoptotic Cell Death.Int J Mol Sci. 2020 Apr 10;21(7):2649. doi: 10.3390/ijms21072649. Int J Mol Sci. 2020. PMID: 32290325 Free PMC article.
-
Synergistic antiproliferative effect of arsenic trioxide combined with bortezomib in HL60 cell line and primary blasts from patients affected by myeloproliferative disorders.Cancer Genet Cytogenet. 2010 Jun;199(2):110-20. doi: 10.1016/j.cancergencyto.2010.02.010. Cancer Genet Cytogenet. 2010. PMID: 20471514
Cited by
-
Natural products as potential drug treatments for acute promyelocytic leukemia.Chin Med. 2024 Apr 3;19(1):57. doi: 10.1186/s13020-024-00928-8. Chin Med. 2024. PMID: 38566147 Free PMC article. Review.
-
The Protective Role of Resveratrol against Arsenic Trioxide-Induced Cardiotoxicity.Evid Based Complement Alternat Med. 2013;2013:407839. doi: 10.1155/2013/407839. Epub 2013 Nov 14. Evid Based Complement Alternat Med. 2013. PMID: 24327821 Free PMC article.
-
Protective effect of resveratrol on arsenic trioxide-induced nephrotoxicity in rats.Nutr Res Pract. 2014 Apr;8(2):220-6. doi: 10.4162/nrp.2014.8.2.220. Epub 2014 Mar 28. Nutr Res Pract. 2014. PMID: 24741408 Free PMC article.
-
Fucoidan enhances the therapeutic potential of arsenic trioxide and all-trans retinoic acid in acute promyelocytic leukemia, in vitro and in vivo.Oncotarget. 2016 Jul 19;7(29):46028-46041. doi: 10.18632/oncotarget.10016. Oncotarget. 2016. PMID: 27329592 Free PMC article.
References
-
- Zhu J, Chen Z, Lallemand-Breitenbach V, De Thé H. How acute promyclocytic leukaemia revived arsenic. Nature Reviews Cancer. 2002;2(9):705–713. - PubMed
-
- Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111(5):2505–2515. - PubMed
-
- Tallman MS. What is the role of arsenic in newly diagnosed APL? Best Practice and Research. 2008;21(4):659–666. - PubMed
-
- Tallman MS, Altman JK. Curative strategies in acute promyelocytic leukemia. Hematology. 2008:391–399. - PubMed
-
- Miller WH, Jr., Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Research. 2002;62(14):3893–3903. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

