A randomized trial comparing the effect of two phone-based interventions on colorectal cancer screening adherence
- PMID: 21826576
- PMCID: PMC3232176
- DOI: 10.1007/s12160-011-9291-z
A randomized trial comparing the effect of two phone-based interventions on colorectal cancer screening adherence
Abstract
Background: Early-stage diagnosis of colorectal cancer is associated with high survival rates; screening prevalence, however, remains suboptimal.
Purpose: This study seeks to test the hypothesis that participants receiving telephone-based tailored education or motivational interviewing had higher colorectal cancer screening completion rates compared to usual care.
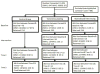
Methods: Primary care patients not adherent with colorectal cancer screening and with no personal or family history of cancer (n = 515) were assigned by block randomization to control (n = 169), tailored education (n = 168), or motivational interview (n = 178). The response rate was 70%; attrition was 24%.
Results: Highest screening occurred in the tailored education group (23.8%, p < .02); participants had 2.2 times the odds of completing a post-intervention colorectal cancer screening than did the control group (AOR = 2.2, CI = 1.2-4.0). Motivational interviewing was not associated with significant increase in post-intervention screening.
Conclusions: Tailored education showed promise as a feasible strategy to increase colorectal cancer screening.
Trial registration: ClinicalTrials.gov NCT01099826.
Conflict of interest statement
Authors have no conflicts of interest to disclose.
Figures
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. - PubMed
-
- Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US multi-society task force on colorectal cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–1595. - PubMed
-
- U.S. Cancer Statistics Working Group. [Accessibility verified January 10, 2010];United States cancer statistics: 1999–2005 incidence and mortality web-based report. Available at http:www.cdc.gov/uscs.
-
- United States Preventive Services Task Force. 2009 clinical guidelines: screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Prev Med. 2009;149:627–637. - PubMed