Multicomponent macrocyclization reactions (MCMRs) employing highly reactive acyl ketene and nitrile oxide intermediates
- PMID: 21827181
- PMCID: PMC3162989
- DOI: 10.1021/ol202024a
Multicomponent macrocyclization reactions (MCMRs) employing highly reactive acyl ketene and nitrile oxide intermediates
Abstract
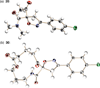
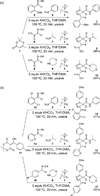
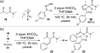
An efficient synthesis of spiro-fused macrolactams by a multicomponent macrocyclization reaction (MCMR) is reported. The use of highly reactive, transient intermediates in this MCMR permits short reaction times, even at high dilution. The methods employed for this MCMR were first developed as a four-component strategy for the synthesis of β-ketoamide isoxazolines and a new macrocyclization reaction is reported.
Figures
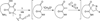




Similar articles
-
Intramolecular cycloaddition reactions of cis-1,2-dihydrocatechol derivatives incorporating C3-tethered diazoketones, nitrile oxides, and azides: stereocontrolled routes to enantiomerically pure spiro[5.5]undecanes and related systems.J Org Chem. 2013 Jul 19;78(14):7100-11. doi: 10.1021/jo400952u. Epub 2013 Jul 1. J Org Chem. 2013. PMID: 23718820
-
Asymmetric synthesis of spiro[isoxazolin-3,3'-oxindoles] via the catalytic 1,3-dipolar cycloaddition reaction of nitrile oxides.J Org Chem. 2014 Aug 15;79(16):7703-10. doi: 10.1021/jo5012625. Epub 2014 Aug 4. J Org Chem. 2014. PMID: 25054839
-
Catalytic, enantioselective 1,3-dipolar cycloadditions of nitrile imines with methyleneindolinones.Org Biomol Chem. 2013 Dec 7;11(45):7834-7. doi: 10.1039/c3ob41815d. Epub 2013 Oct 17. Org Biomol Chem. 2013. PMID: 24132663 Free PMC article.
-
Catalytic Enantioselective [3 + 2] Cycloaddition of α-Keto Ester Enolates and Nitrile Oxides.J Am Chem Soc. 2017 Jun 28;139(25):8661-8666. doi: 10.1021/jacs.7b03782. Epub 2017 Jun 15. J Am Chem Soc. 2017. PMID: 28581747 Free PMC article.
-
One-Pot Conversion of N-Allyl-α-cyano Esters to α-Allyl-α-cyano Lactams through a Hydrolysis/Ketene Formation/Cyclization/Claisen Rearrangement Sequence.Org Lett. 2016 Mar 4;18(5):889-91. doi: 10.1021/acs.orglett.5b02843. Epub 2016 Feb 12. Org Lett. 2016. PMID: 26872217
Cited by
-
Expedient synthesis of a 72-membered isoxazolino-β-ketoamide library by a 2·3-component reaction.ACS Comb Sci. 2012 Feb 13;14(2):85-8. doi: 10.1021/co200199h. Epub 2012 Jan 3. ACS Comb Sci. 2012. PMID: 22181856 Free PMC article.
References
-
- Shu YZ. J. Nat. Prod. 1998;61:1053–1071. - PubMed
- Choi K, Hamilton AD. J. Am. Chem. Soc. 2001;123:2456–5457. - PubMed
- Fernández-López S, Kim H-S, Choi EC, Delgado M, Granja JR, Khasanov A, Kraehenbuehl K, Long G, Weinberger DA, Wilcoxen KM, Ghadiri MR. Nature. 2001;412:452–455. - PubMed
- Loughlin WA, Tyndall JDA, Glenn MP, Fairlie DP. Chem. Rev. 2004;104:6085–6117. - PubMed
- Oyelere AK. Curr. Top. Med. Chem. 2010;10:1359–1360. - PubMed
-
- de Vega MJP, Martín-Martínez M, González-Muñiz R. Curr. Top. Med. Chem. 2007;7:33–62. - PubMed
- Peczuh MW, Hamilton AD. Chem. Rev. 2000;100:2479–2494. - PubMed
- Leon F, Rivera DG, Wessjohann LA. J. Org. Chem. 2008;73:1762–1767. - PubMed
- Tyndall JDA, Fairlie DP. Curr. Med. Chem. 2001;8:893–907. - PubMed
-
- Janvier P, Bois-Choussy M, Bienayme H, Zhu J. Angew. Chem. Int. Ed. 2003;42:811–814. - PubMed
- Zhao G, Sun X, Bienayme H, Zhu J. J. Am. Chem. Soc. 2001;123:6700–6701. - PubMed
- Pirali T, Tron GC, Zhu J. Org. Lett. 2006;8:4145–4148. - PubMed
- Bughin C, Masson G, Zhu J. J. Org. Chem. 2007;72:1826–1829. - PubMed
-
- Michalik D, Schaks A, Wessjohann LA. Eur. J. Org. Chem. 2007:149–157.
- Rivera DG, Wessjohann LA. J. Am. Chem. Soc. 2009;131:3721–3732. - PubMed
- Rivera DG, Wessjohann LA. J. Am. Chem. Soc. 2006;128:7122–7123. - PubMed
- Leon F, Rivera DG, Wessjohann LA. J. Org. Chem. 2008;73:1762–1767. - PubMed
- Rivera DG, Pando O, Bosch R, Wessjohann LA. J. Org. Chem. 2008;73:6229–6238. - PubMed
- Wessjohaan LA, Rivera DG, Coll F. J. Org. Chem. 2006;71:7521–7526. - PubMed
- Wessjohann LA, Rivera DG, Leon F. Org. Lett. 2007;9:4733–4736. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

