Corticosteroid-induced gene expression in allergen-challenged asthmatic subjects taking inhaled budesonide
- PMID: 21827450
- PMCID: PMC3372826
- DOI: 10.1111/j.1476-5381.2011.01620.x
Corticosteroid-induced gene expression in allergen-challenged asthmatic subjects taking inhaled budesonide
Abstract
Background and purpose: Inhaled corticosteroids (ICS) are the cornerstone of asthma pharmacotherapy and, acting via the glucocorticoid receptor (GR), reduce inflammatory gene expression. While this is often attributed to a direct inhibitory effect of the GR on inflammatory gene transcription, corticosteroids also induce the expression of anti-inflammatory genes in vitro. As there are no data to support this effect in asthmatic subjects taking ICS, we have assessed whether ICS induce anti-inflammatory gene expression in subjects with atopic asthma.
Experimental approach: Bronchial biopsies from allergen-challenged atopic asthmatic subjects taking inhaled budesonide or placebo were subjected to gene expression analysis using real-time reverse transcriptase-PCR for the corticosteroid-inducible genes (official gene symbols with aliases in parentheses): TSC22D3 [glucocorticoid-induced leucine zipper (GILZ)], dual-specificity phosphatase-1 (MAPK phosphatase-1), both anti-inflammatory effectors, and FKBP5 [FK506-binding protein 51 (FKBP51)], a regulator of GR function. Cultured pulmonary epithelial and smooth muscle cells were also treated with corticosteroids before gene expression analysis.
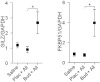
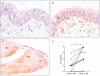
Key results: Compared with placebo, GILZ and FKBP51 mRNA expression was significantly elevated in budesonide-treated subjects. Budesonide also increased GILZ expression in human epithelial and smooth muscle cells in culture. Immunostaining of bronchial biopsies revealed GILZ expression in the airways epithelium and smooth muscle of asthmatic subjects.
Conclusions and implications: Expression of the corticosteroid-induced genes, GILZ and FKBP51, is up-regulated in the airways of allergen-challenged asthmatic subjects taking inhaled budesonide. Consequently, the biological effects of corticosteroid-induced genes should be considered when assessing the actions of ICS. Treatment modalities that increase or decrease GR-dependent transcription may correspondingly affect corticosteroid efficacy.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures


Similar articles
-
Comparison of Symbicort® versus Pulmicort® on steroid pharmacodynamic markers in asthma patients.Respir Med. 2011 Dec;105(12):1784-9. doi: 10.1016/j.rmed.2011.08.020. Epub 2011 Sep 7. Respir Med. 2011. PMID: 21903370 Clinical Trial.
-
Comparison of 3 different doses of budesonide and placebo on the early asthmatic response to inhaled allergen.J Allergy Clin Immunol. 1998 Sep;102(3):363-7. doi: 10.1016/s0091-6749(98)70121-6. J Allergy Clin Immunol. 1998. PMID: 9768574 Clinical Trial.
-
Evaluation of single-dose inhaled corticosteroid activity with an allergen challenge model.J Allergy Clin Immunol. 1997 Jul;100(1):65-70. doi: 10.1016/s0091-6749(97)70196-9. J Allergy Clin Immunol. 1997. PMID: 9257789 Clinical Trial.
-
New Versus Old: The Impact of Changing Patterns of Inhaled Corticosteroid Prescribing and Dosing Regimens in Asthma Management.Adv Ther. 2022 May;39(5):1895-1914. doi: 10.1007/s12325-022-02092-7. Epub 2022 Mar 14. Adv Ther. 2022. PMID: 35284999 Free PMC article. Review.
-
Inhaled corticosteroids as combination therapy with beta-adrenergic agonists in airways disease: present and future.Eur J Clin Pharmacol. 2009 Sep;65(9):853-71. doi: 10.1007/s00228-009-0682-z. Epub 2009 Jun 26. Eur J Clin Pharmacol. 2009. PMID: 19557399 Review.
Cited by
-
Dexamethasone inhibits repair of human airway epithelial cells mediated by glucocorticoid-induced leucine zipper (GILZ).PLoS One. 2013;8(4):e60705. doi: 10.1371/journal.pone.0060705. Epub 2013 Apr 3. PLoS One. 2013. PMID: 23573276 Free PMC article.
-
Airway Smooth Muscle-Specific Transcriptomic Signatures of Glucocorticoid Exposure.Am J Respir Cell Mol Biol. 2019 Jul;61(1):110-120. doi: 10.1165/rcmb.2018-0385OC. Am J Respir Cell Mol Biol. 2019. PMID: 30694689 Free PMC article.
-
Inhaled corticosteroids do not reduce initial high activity of matrix metalloproteinase (MMP)-9 in exhaled breath condensates of children with asthma exacerbation: a proof of concept study.Cent Eur J Immunol. 2016;41(2):221-7. doi: 10.5114/ceji.2016.60998. Epub 2016 Jul 15. Cent Eur J Immunol. 2016. PMID: 27536209 Free PMC article.
-
Potential mechanisms to explain how LABAs and PDE4 inhibitors enhance the clinical efficacy of glucocorticoids in inflammatory lung diseases.F1000Prime Rep. 2015 Feb 3;7:16. doi: 10.12703/P7-16. eCollection 2015. F1000Prime Rep. 2015. PMID: 25750734 Free PMC article. Review.
-
Understanding how long-acting β2 -adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids in asthma - an update.Br J Pharmacol. 2016 Dec;173(24):3405-3430. doi: 10.1111/bph.13628. Epub 2016 Nov 9. Br J Pharmacol. 2016. PMID: 27646470 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

