Hypoxia-mimetic agents inhibit proliferation and alter the morphology of human umbilical cord-derived mesenchymal stem cells
- PMID: 21827650
- PMCID: PMC3166919
- DOI: 10.1186/1471-2121-12-32
Hypoxia-mimetic agents inhibit proliferation and alter the morphology of human umbilical cord-derived mesenchymal stem cells
Abstract
Background: The therapeutic efficacy of human mesenchymal stem cells (hMSCs) for the treatment of hypoxic-ischemic diseases is closely related to level of hypoxia in the damaged tissues. To elucidate the potential therapeutic applications and limitations of hMSCs derived from human umbilical cords, the effects of hypoxia on the morphology and proliferation of hMSCs were analyzed.
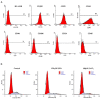
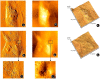
Results: After treatment with DFO and CoCl₂, hMSCs were elongated, and adjacent cells were no longer in close contact. In addition, vacuole-like structures were observed within the cytoplasm; the rough endoplasmic reticulum expanded, and expanded ridges were observed in mitochondria. In addition, DFO and CoCl₂ treatments for 48 h significantly inhibited hMSCs proliferation in a concentration-dependent manner (P < 0.05). This treatment also increased the number of cells in G0/G1 phase and decreased those in G2/S/M phase.
Conclusions: The hypoxia-mimetic agents, DFO and CoCl₂, alter umbilical cord-derived hMSCs morphology and inhibit their proliferation through influencing the cell cycle.
Figures


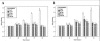


References
-
- Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues, Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–40. doi: 10.1097/00007890-197404000-00001. - DOI - PubMed
-
- Alviano F, Fossati V, Marchionni C, Arpinati M, Bonsi L, Franchina M, Lanzoni G, Cantoni S, Cavallini C, Bianchi F, Tazzari PL, Pasquinelli G, Foroni L, Ventura C, Grossi A, Bagnara GP. Term Amniotic membrane is a high throughput source for multipotent Mesenchymal Stem Cells with the ability to differentiate into endothelial cells in vitro. BMC Dev Biol. 2007;7:1–14. doi: 10.1186/1471-213X-7-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

