Increased hydrophobicity and decreased backbone flexibility explain the lower solubility of a cataract-linked mutant of γD-crystallin
- PMID: 21827768
- PMCID: PMC3184788
- DOI: 10.1016/j.jmb.2011.07.058
Increased hydrophobicity and decreased backbone flexibility explain the lower solubility of a cataract-linked mutant of γD-crystallin
Abstract
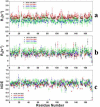
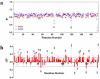
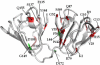
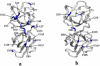
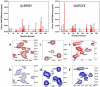
A number of point mutations in γD-crystallin are associated with human cataract. The Pro23-to-Thr (P23T) mutation is perhaps the most common, is geographically widespread, and presents itself in a variety of phenotypes. It is therefore important to understand the molecular basis of lens opacity due to this mutation. In our earlier studies, we noted that P23T shows retrograde and sharply lowered solubility, most likely due to the emergence of hydrophobic patches involved in protein aggregation. Binding of 4,4'-dianilino-1,1'-binaphthyl-5,5'-disulfonate (Bis-ANS) dye (a probe commonly used for detecting surface hydrophobicity) competed with aggregation, suggesting that the residues involved in Bis-ANS binding are also involved in protein aggregation. Here, using NMR spectroscopy in conjunction with Bis-ANS binding, we identify three residues (Y16, D21, and Y50) in P23T that are involved in binding the dye. Furthermore, using (15)N NMR relaxation experiments, we show that, in the mutant protein, backbone fluctuations are restricted to the picosecond-to-nanosecond and microsecond timescales relative to the wild type. Our present studies specify the residues involved in these two pivotal characteristics of the mutant protein, namely increased surface hydrophobicity and restricted mobility of the protein backbone, which can explain the nucleation and further propagation of protein aggregates. Thus, we have now identified the residues in the P23T mutant that give rise to novel hydrophobic surfaces, as well as those regions of the protein backbone where fluctuations in different timescales are restricted, providing a comprehensive understanding of how lens opacity could result from this mutation.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
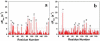
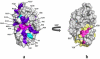
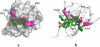
References
-
- Zetterstrom C, Lundvall A, Kugelberg M. Cataracts in children. J Cataract Refract Surg. 2005;31:824–40. - PubMed
-
- Hammond CJ, Snieder H, Spector TD, Gilbert CE. Genetic and environmental factors in age-related nuclear cataracts in monozygotic and dizygotic twins. N Engl J Med. 2000;342:1786–90. - PubMed
-
- Pande A, Annunziata O, Asherie N, Ogun O, Benedek GB, Pande J. Decrease in protein solubility and cataract formation caused by the Pro23 to Thr mutation in human gamma D-crystallin. Biochemistry. 2005;44:2491–500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

