Systemic augmentation of alphaB-crystallin provides therapeutic benefit twelve hours post-stroke onset via immune modulation
- PMID: 21828004
- PMCID: PMC3156222
- DOI: 10.1073/pnas.1107368108
Systemic augmentation of alphaB-crystallin provides therapeutic benefit twelve hours post-stroke onset via immune modulation
Abstract
Tissue plasminogen activator is the only treatment option for stroke victims; however, it has to be administered within 4.5 h after symptom onset, making its use very limited. This report describes a unique target for effective treatment of stroke, even 12 h after onset, by the administration of αB-crystallin (Cryab), an endogenous immunomodulatory neuroprotectant. In Cryab(-/-) mice, there was increased lesion size and diminished neurologic function after stroke compared with wild-type mice. Increased plasma Cryab was detected after experimental stroke in mice and after stroke in human patients. Administration of Cryab even 12 h after experimental stroke reduced both stroke volume and inflammatory cytokines associated with stroke pathology. Cryab is an endogenous anti-inflammatory and neuroprotectant molecule produced after stroke, whose beneficial properties can be augmented when administered therapeutically after stroke.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
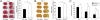
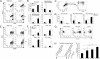

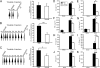

References
-
- Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. - PubMed
-
- Lees KR, et al. ECASS, ATLANTIS, NINDS and EPITHET rt-PA Study Group Time to treatment with intravenous alteplase and outcome in stroke: An updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. - PubMed
-
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

