Contractile forces contribute to increased glycosylphosphatidylinositol-anchored receptor CD24-facilitated cancer cell invasion
- PMID: 21828044
- PMCID: PMC3186419
- DOI: 10.1074/jbc.M111.245183
Contractile forces contribute to increased glycosylphosphatidylinositol-anchored receptor CD24-facilitated cancer cell invasion
Abstract
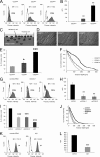
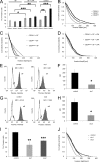
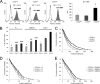
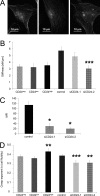
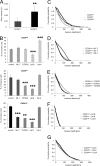
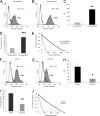
The malignancy of a tumor depends on the capability of cancer cells to metastasize. The process of metastasis involves cell invasion through connective tissue and transmigration through endothelial monolayers. The expression of the glycosylphosphatidylinositol-anchored receptor CD24 is increased in several tumor types and is consistently associated with increased metastasis formation in patients. Furthermore, the localization of β1-integrins in lipid rafts depends on CD24. Cell invasion is a fundamental biomechanical process and usually requires cell adhesion to the extracellular matrix (ECM) mainly through β1 heterodimeric integrin receptors. Here, we studied the invasion of A125 human lung cancer cells with different CD24 expression levels in three-dimensional ECMs. We hypothesized that CD24 expression increases cancer cell invasion through increased contractile forces. To analyze this, A125 cells (CD24 negative) were stably transfected with CD24 and sorted for high and low CD24 expression. The invasiveness of the CD24(high) and CD24(low) transfectants was determined in three-dimensional ECMs. The percentage of invasive cells and their invasion depth was increased in CD24(high) cells compared with CD24(low) cells. Knockdown of CD24 and of the β1-integrin subunit in CD24(high) cells decreased their invasiveness, indicating that the increased invasiveness is CD24- and β1-integrin subunit-dependent. Fourier transform traction microscopy revealed that the CD24(high) cells generated 5-fold higher contractile forces compared with CD24(low) cells. To analyze whether contractile forces are essential for CD24-facilitated cell invasion, we performed invasion assays in the presence of myosin light chain kinase inhibitor ML-7 as well as Rho kinase inhibitor Y27632. Cell invasiveness was reduced after addition of ML-7 and Y27632 in CD24(high) cells but not in CD24(neg) cells. Moreover, after addition of lysophosphatidic acid or calyculin A, an increase in pre-stress in CD24(neg) cells was observed, which enhanced cellular invasiveness. In addition, inhibition of the Src kinase or STAT3 strongly reduced the invasiveness of CD24(high) cells, slightly reduced that of CD24(low) cells, and did not alter the invasiveness of CD24(neg) cells. Taken together, these results suggest that CD24 enhances cell invasion through increased generation or transmission of contractile forces.
Figures

Similar articles
-
Integrin α5β1 facilitates cancer cell invasion through enhanced contractile forces.J Cell Sci. 2011 Feb 1;124(Pt 3):369-83. doi: 10.1242/jcs.071985. Epub 2011 Jan 11. J Cell Sci. 2011. PMID: 21224397 Free PMC article.
-
Phagocytized beads reduce the α5β1 integrin facilitated invasiveness of cancer cells by regulating cellular stiffness.Cell Biochem Biophys. 2013 Jul;66(3):599-622. doi: 10.1007/s12013-012-9506-3. Cell Biochem Biophys. 2013. PMID: 23329175
-
CD24 interacts with and promotes the activity of c-src within lipid rafts in breast cancer cells, thereby increasing integrin-dependent adhesion.Cell Mol Life Sci. 2012 Feb;69(3):435-48. doi: 10.1007/s00018-011-0756-9. Epub 2011 Jun 28. Cell Mol Life Sci. 2012. PMID: 21710320 Free PMC article.
-
The matrix environmental and cell mechanical properties regulate cell migration and contribute to the invasive phenotype of cancer cells.Rep Prog Phys. 2019 Jun;82(6):064602. doi: 10.1088/1361-6633/ab1628. Epub 2019 Apr 4. Rep Prog Phys. 2019. PMID: 30947151 Review.
-
Contractile forces in tumor cell migration.Eur J Cell Biol. 2008 Sep;87(8-9):669-76. doi: 10.1016/j.ejcb.2008.01.002. Epub 2008 Mar 4. Eur J Cell Biol. 2008. PMID: 18295931 Free PMC article. Review.
Cited by
-
The natural anticancer agent cantharidin alters GPI-anchored protein sorting by targeting Cdc1-mediated remodeling in endoplasmic reticulum.J Biol Chem. 2019 Mar 15;294(11):3837-3852. doi: 10.1074/jbc.RA118.003890. Epub 2019 Jan 18. J Biol Chem. 2019. PMID: 30659098 Free PMC article.
-
Membranous CD24 expression as detected by the monoclonal antibody SWA11 is a prognostic marker in non-small cell lung cancer patients.BMC Clin Pathol. 2015 Nov 16;15:19. doi: 10.1186/s12907-015-0019-z. eCollection 2015. BMC Clin Pathol. 2015. PMID: 26578846 Free PMC article.
-
Decoding CD24: Roles of chemoradiotherapy resistance and potential as therapeutic targets.Oncol Res. 2025 May 29;33(6):1347-1361. doi: 10.32604/or.2025.059327. eCollection 2025. Oncol Res. 2025. PMID: 40486886 Free PMC article. Review.
-
Curcumin inhibits LPA-induced invasion by attenuating RhoA/ROCK/MMPs pathway in MCF7 breast cancer cells.Clin Exp Med. 2016 Feb;16(1):37-47. doi: 10.1007/s10238-015-0336-7. Epub 2015 Jan 18. Clin Exp Med. 2016. PMID: 25596714
-
CD24 promotes tumor cell invasion by suppressing tissue factor pathway inhibitor-2 (TFPI-2) in a c-Src-dependent fashion.Clin Exp Metastasis. 2012 Jan;29(1):27-38. doi: 10.1007/s10585-011-9426-4. Epub 2011 Oct 8. Clin Exp Metastasis. 2012. PMID: 21984372
References
-
- Liotta L. A., Steeg P. S., Stetler-Stevenson W. G. (1991) Cell 64, 327–336 - PubMed
-
- Al-Mehdi A. B., Tozawa K., Fisher A. B., Shientag L., Lee A., Muschel R. J. (2000) Nat. Med. 6, 100–102 - PubMed
-
- Steeg P. S. (2006) Nat. Med. 12, 895–904 - PubMed
-
- Langley R. R., Fidler I. J. (2007) Endocr. Rev. 28, 297–321 - PubMed
-
- Horwitz A. F. (1997) Sci. Am. 276, 68–75 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

