Proteolipidic composition of exosomes changes during reticulocyte maturation
- PMID: 21828046
- PMCID: PMC3190795
- DOI: 10.1074/jbc.M111.257444
Proteolipidic composition of exosomes changes during reticulocyte maturation
Abstract
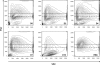
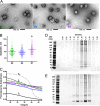
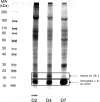
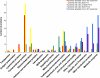
During the orchestrated process leading to mature erythrocytes, reticulocytes must synthesize large amounts of hemoglobin, while eliminating numerous cellular components. Exosomes are small secreted vesicles that play an important role in this process of specific elimination. To understand the mechanisms of proteolipidic sorting leading to their biogenesis, we have explored changes in the composition of exosomes released by reticulocytes during their differentiation, in parallel to their physical properties. By combining proteomic and lipidomic approaches, we found dramatic alterations in the composition of the exosomes retrieved over the course of a 7-day in vitro differentiation protocol. Our data support a previously proposed model, whereby in reticulocytes the biogenesis of exosomes involves several distinct mechanisms for the preferential recruitment of particular proteins and lipids and suggest that the respective prominence of those pathways changes over the course of the differentiation process.
Figures
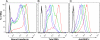

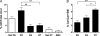

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

