Pericellular versican regulates the fibroblast-myofibroblast transition: a role for ADAMTS5 protease-mediated proteolysis
- PMID: 21828051
- PMCID: PMC3190794
- DOI: 10.1074/jbc.M111.254938
Pericellular versican regulates the fibroblast-myofibroblast transition: a role for ADAMTS5 protease-mediated proteolysis
Abstract
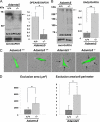
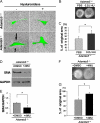
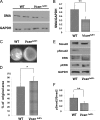
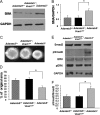
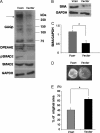
The cell and its glycosaminoglycan-rich pericellular matrix (PCM) comprise a functional unit. Because modification of PCM influences cell behavior, we investigated molecular mechanisms that regulate PCM volume and composition. In fibroblasts and other cells, aggregates of hyaluronan and versican are found in the PCM. Dermal fibroblasts from Adamts5(-/-) mice, which lack a versican-degrading protease, ADAMTS5, had reduced versican proteolysis, increased PCM, altered cell shape, enhanced α-smooth muscle actin (SMA) expression and increased contractility within three-dimensional collagen gels. The myofibroblast-like phenotype was associated with activation of TGFβ signaling. We tested the hypothesis that fibroblast-myofibroblast transition in Adamts5(-/-) cells resulted from versican accumulation in PCM. First, we noted that versican overexpression in human dermal fibroblasts led to increased SMA expression, enhanced contractility, and increased Smad2 phosphorylation. In contrast, dermal fibroblasts from Vcan haploinsufficient (Vcan(hdf/+)) mice had reduced contractility relative to wild type fibroblasts. Using a genetic approach to directly test if myofibroblast transition in Adamts5(-/-) cells resulted from increased PCM versican content, we generated Adamts5(-/-);Vcan(hdf/+) mice and isolated their dermal fibroblasts for comparison with dermal fibroblasts from Adamts5(-/-) mice. In Adamts5(-/-) fibroblasts, Vcan haploinsufficiency or exogenous ADAMTS5 restored normal fibroblast contractility. These findings demonstrate that altering PCM versican content through proteolytic activity of ADAMTS5 profoundly influenced the dermal fibroblast phenotype and may regulate a phenotypic continuum between the fibroblast and its alter ego, the myofibroblast. We propose that a physiological function of ADAMTS5 in dermal fibroblasts is to maintain optimal versican content and PCM volume by continually trimming versican in hyaluronan-versican aggregates.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

