Effective fiber hypertrophy in satellite cell-depleted skeletal muscle
- PMID: 21828094
- PMCID: PMC3152923
- DOI: 10.1242/dev.068858
Effective fiber hypertrophy in satellite cell-depleted skeletal muscle
Abstract
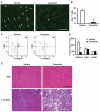
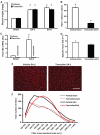
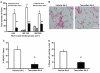
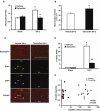
An important unresolved question in skeletal muscle plasticity is whether satellite cells are necessary for muscle fiber hypertrophy. To address this issue, a novel mouse strain (Pax7-DTA) was created which enabled the conditional ablation of >90% of satellite cells in mature skeletal muscle following tamoxifen administration. To test the hypothesis that satellite cells are necessary for skeletal muscle hypertrophy, the plantaris muscle of adult Pax7-DTA mice was subjected to mechanical overload by surgical removal of the synergist muscle. Following two weeks of overload, satellite cell-depleted muscle showed the same increases in muscle mass (approximately twofold) and fiber cross-sectional area with hypertrophy as observed in the vehicle-treated group. The typical increase in myonuclei with hypertrophy was absent in satellite cell-depleted fibers, resulting in expansion of the myonuclear domain. Consistent with lack of nuclear addition to enlarged fibers, long-term BrdU labeling showed a significant reduction in the number of BrdU-positive myonuclei in satellite cell-depleted muscle compared with vehicle-treated muscle. Single fiber functional analyses showed no difference in specific force, Ca(2+) sensitivity, rate of cross-bridge cycling and cooperativity between hypertrophied fibers from vehicle and tamoxifen-treated groups. Although a small component of the hypertrophic response, both fiber hyperplasia and regeneration were significantly blunted following satellite cell depletion, indicating a distinct requirement for satellite cells during these processes. These results provide convincing evidence that skeletal muscle fibers are capable of mounting a robust hypertrophic response to mechanical overload that is not dependent on satellite cells.
Figures
References
-
- Adams G. R., Caiozzo V. J., Haddad F., Baldwin K. M. (2002). Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am. J. Physiol. Cell Physiol. 283, C1182-C1195 - PubMed
-
- Akiho M., Nakashima H., Sakata M., Yamasa Y., Yamaguchi A., Sakuma K. (2010). Expression profile of Notch-1 in mechanically overloaded plantaris muscle of mice. Life Sci. 86, 59-65 - PubMed
-
- Blaauw B., Canato M., Agatea L., Toniolo L., Mammucari C., Masiero E., Abraham R., Sandri M., Schiaffino S., Reggiani C. (2009). Inducible activation of Akt increases skeletal muscle mass and force without satellite cell activation. FASEB J. 23, 3896-3905 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

