Extracellular matrix structure and nano-mechanics determine megakaryocyte function
- PMID: 21828129
- PMCID: PMC3291488
- DOI: 10.1182/blood-2011-04-345876
Extracellular matrix structure and nano-mechanics determine megakaryocyte function
Abstract
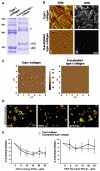
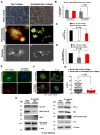
Cell interactions with matrices via specific receptors control many functions, with chemistry, physics, and membrane elasticity as fundamental elements of the processes involved. Little is known about how biochemical and biophysical processes integrate to generate force and, ultimately, to regulate hemopoiesis into the bone marrow-matrix environment. To address this hypothesis, in this work we focus on the regulation of MK development by type I collagen. By atomic force microscopy analysis, we demonstrate that the tensile strength of fibrils in type I collagen structure is a fundamental requirement to regulate cytoskeleton contractility of human MKs through the activation of integrin-α2β1-dependent Rho-ROCK pathway and MLC-2 phosphorylation. Most importantly, this mechanism seemed to mediate MK migration, fibronectin assembly, and platelet formation. On the contrary, a decrease in mechanical tension caused by N-acetylation of lysine side chains in type I collagen completely reverted these processes by preventing fibrillogenesis.
Figures
References
-
- Chang Y, Aurade F, Larbret F, et al. Proplatelet formation is regulated by the Rho/ROCK pathway. Blood. 2007;109(10):4229–4236. - PubMed
-
- Spurlin TA, Bhadriraju K, Chung KH, et al. The treatment of collagen fibrils by tissue transglutaminase to promote vascular smooth muscle cell contractile signaling. Biomaterials. 2009;30(29):5486–5496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

