TGF-β1 signaling and Krüppel-like factor 10 regulate bone marrow-derived proangiogenic cell differentiation, function, and neovascularization
- PMID: 21828131
- PMCID: PMC3236126
- DOI: 10.1182/blood-2011-06-363713
TGF-β1 signaling and Krüppel-like factor 10 regulate bone marrow-derived proangiogenic cell differentiation, function, and neovascularization
Abstract
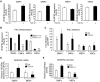
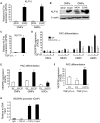
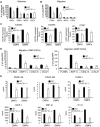
Emerging evidence demonstrates that proangiogenic cells (PACs) originate from the BM and are capable of being recruited to sites of ischemic injury where they contribute to neovascularization. We previously determined that among hematopoietic progenitor stem cells, common myeloid progenitors (CMPs) and granulocyte-macrophage progenitor cells (GMPs) differentiate into PACs and possess robust angiogenic activity under ischemic conditions. Herein, we report that a TGF-β1-responsive Krüppel- like factor, KLF10, is strongly expressed in PACs derived from CMPs and GMPs, ∼ 60-fold higher than in progenitors lacking PAC markers. KLF10(-/-) mice present with marked defects in PAC differentiation, function, TGF-β responsiveness, and impaired blood flow recovery after hindlimb ischemia, an effect rescued by wild-type PACs, but not KLF10(-/-) PACs. Overexpression studies revealed that KLF10 could rescue PAC formation from TGF-β1(+/-) CMPs and GMPs. Mechanistically, KLF10 targets the VEGFR2 promoter in PACs which may underlie the observed effects. These findings may be clinically relevant because KLF10 expression was also found to be significantly reduced in PACs from patients with peripheral artery disease. Collectively, these observations identify TGF-β1 signaling and KLF10 as key regulators of functional PACs derived from CMPs and GMPs and may provide a therapeutic target during cardiovascular ischemic states.
Figures

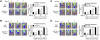

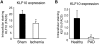
References
-
- Burt RK, Loh Y, Pearce W, et al. Clinical applications of blood-derived and marrow-derived stem cells for nonmalignant diseases [see comment]. JAMA. 2008;299(8):925–936. - PubMed
-
- Fadini GP, Sartore S, Albiero M, et al. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 2006;26(9):2140–2146. - PubMed
-
- Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89(1):E1–7. - PubMed
-
- Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk [see comment]. [reprint in Can J Cardiol. 2004;20(suppl B):44B-48B]. N Engl J Med. 2003;348(7):593–600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 DE014036/DE/NIDCR NIH HHS/United States
- HL091076/HL/NHLBI NIH HHS/United States
- HL080174/HL/NHLBI NIH HHS/United States
- HL075771/HL/NHLBI NIH HHS/United States
- R01 HL091076/HL/NHLBI NIH HHS/United States
- P30 DK057521/DK/NIDDK NIH HHS/United States
- F32 HL088819/HL/NHLBI NIH HHS/United States
- F32HL088819/HL/NHLBI NIH HHS/United States
- R01 DE014036/DE/NIDCR NIH HHS/United States
- P30DK057521/DK/NIDDK NIH HHS/United States
- DE14036/DE/NIDCR NIH HHS/United States
- R01 HL080174/HL/NHLBI NIH HHS/United States
- R01 HL075771/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

