Role of ZBP-89 in human globin gene regulation and erythroid differentiation
- PMID: 21828133
- PMCID: PMC3186340
- DOI: 10.1182/blood-2011-03-341446
Role of ZBP-89 in human globin gene regulation and erythroid differentiation
Abstract
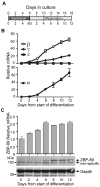
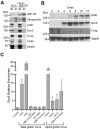
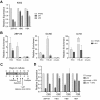
The molecular mechanisms underlying erythroid-specific gene regulation remain incompletely understood. Closely spaced binding sites for GATA, NF-E2/maf, and CACCC interacting transcription factors play functionally important roles in globin and other erythroid-specific gene expression. We and others recently identified the CACCC-binding transcription factor ZBP-89 as a novel GATA-1 and NF-E2/mafK interacting partner. Here, we examined the role of ZBP-89 in human globin gene regulation and erythroid maturation using a primary CD34(+) cell ex vivo differentiation system. We show that ZBP-89 protein levels rise dramatically during human erythroid differentiation and that ZBP-89 occupies key cis-regulatory elements within the globin and other erythroid gene loci. ZBP-89 binding correlates strongly with RNA Pol II occupancy, active histone marks, and high-level gene expression. ZBP-89 physically associates with the histone acetyltransferases p300 and Gcn5/Trrap, and occupies common sites with Gcn5 within the human globin loci. Lentiviral short hairpin RNAs knockdown of ZBP-89 results in reduced Gcn5 occupancy, decreased acetylated histone 3 levels, lower globin and erythroid-specific gene expression, and impaired erythroid maturation. Addition of the histone deacetylase inhibitor valproic acid partially reverses the reduced globin gene expression. These findings reveal an activating role for ZBP-89 in human globin gene regulation and erythroid differentiation.
Figures



References
-
- Jamison DT, editor. Priorities in Health. Washington, DC: World Bank Group; 2006.
-
- Harju S, McQueen KJ, Peterson KR. Chromatin structure and control of β-like globin gene switching. Exp Biol Med. 2002;227(9):683–700. - PubMed
-
- Ikuta T, Papayannopoulou T, Stamatoyannopoulos G, Kan YW. Globin gene switching: in vivo protein-DNA interactions of the human beta-globin locus in erythroid cells expressing the fetal or the adult globin gene program. J Biol Chem. 1996;271(24):14082–14091. - PubMed
-
- Donze D, Townes TM, Bieker JJ. Role of erythroid Kruppel-like factor in human gamma- to beta-globin gene switching. J Biol Chem. 1995;270(4):1955–1959. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

