Outcome of AL amyloidosis after high-dose melphalan and autologous stem cell transplantation: long-term results in a series of 421 patients
- PMID: 21828140
- PMCID: PMC3204906
- DOI: 10.1182/blood-2011-01-330738
Outcome of AL amyloidosis after high-dose melphalan and autologous stem cell transplantation: long-term results in a series of 421 patients
Abstract
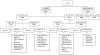
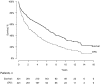
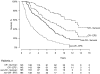
Previous studies have suggested that, in patients with AL amyloidosis treated with high-dose melphalan and autologous stem-cell transplantation (HDM/SCT), the greatest benefit is seen in those patients achieving a hematologic complete response (CR). We analyzed a series of 421 consecutive patients treated with HDM/SCT at a single referral center and compared outcomes for patients with and without CR. Treatment-related mortality was 11.4% overall (5.6% in the last 5 years). By intention-to-treat analysis, the CR rate was 34% and the median event-free survival (EFS) and overall survival (OS) were 2.6 and 6.3 years, respectively. Eighty-one patients died within the first year after HDM/SCT and were not evaluable for hematologic and organ response. Of 340 evaluable patients, 43% achieved CR and 78% of them experienced an organ response. For CR patients, median EFS and OS were 8.3 and 13.2 years, respectively. Among the 195 patients who did not obtain CR, 52% achieved an organ response, and their median EFS and OS were 2 and 5.9 years, respectively. Thus, treatment of selected AL patients with HDM/SCT resulted in a high organ response rate and long OS, even for those patients who did not achieve CR.
Figures
Comment in
-
ASCT for AL: all's well that ends well.Blood. 2011 Oct 20;118(16):4298-9. doi: 10.1182/blood-2011-08-375105. Blood. 2011. PMID: 22021449 No abstract available.
References
-
- Kyle RA, Linos A, Beard CM, et al. Incidence and natural history of primary systemic amiloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood. 1992;79(7):1817–1822. - PubMed
-
- Skinner M, Anderson J, Simms R, et al. Treatment of 100 patients with primary amyloidosis: a randomized trial of melphalan, prednisone, and colchicine vs colchicine only. Am J Med. 1996;100(3):290–298. - PubMed
-
- Kyle RA, Gertz MA, Greipp PR, et al. Long-term survival (10 years or more) in 30 patients with primary amyloidosis. Blood. 1999;93(3):1062–1066. - PubMed
-
- Kyle RA, Gertz MA, Greipp PR, et al. A trial of three regimens for primary amyloidosis: colchicine alone, melphalan and prednisone, and melphalan, prednisone and colchicine. N Engl J Med. 1997;336(17):1202–1207. - PubMed
-
- Gertz MA, Lacy MQ, Lust JA, et al. Prospective randomized trial of melphalan and prednisone vs vincristine, carmustine, melphalan, cyclophosphamide, and prednisone in the treatment of primary systemic amyloidosis. J Clin Oncol. 1999;17(1):262–267. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

