Characterization of a novel potassium-competitive acid blocker of the gastric H,K-ATPase, 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438)
- PMID: 21828261
- PMCID: PMC3199995
- DOI: 10.1124/jpet.111.185314
Characterization of a novel potassium-competitive acid blocker of the gastric H,K-ATPase, 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438)
Abstract
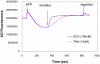
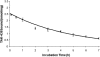
Inhibition of the gastric H,K-ATPase by the potassium-competitive acid blocker (P-CAB) 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine (TAK-438), is strictly K(+)-competitive with a K(i) of 10 nM at pH 7. In contrast to previous P-CABs, this structure has a point positive charge (pK(a) 9.06) allowing for greater accumulation in parietal cells compared with previous P-CABs [e.g., (8-benzyloxy-2-methyl-imidazo(1,2-a)pyridin-3-yl)acetonitrile (SCH28080), pK(a) 5.6]. The dissociation rate of the compound from the isolated ATPase is slower than other P-CABs, with the t(1/2) being 7.5 h in 20 mM KCl at pH 7. The stoichiometry of binding of TAK-438 to the H,K-ATPase is 2.2 nmol/mg in the presence of Mg-ATP, vanadate, or MgP(i). However, TAK-438 also binds enzyme at 1.3 nmol/mg in the absence of Mg(2+). Modeling of the H,K-ATPase to the homologous Na,K-ATPase predicts a close approach and hydrogen bonding between the positively charged N-methylamino group and the negatively charged Glu795 in the K(+)-binding site in contrast to the planar diffuse positive charge of previous P-CABs. This probably accounts for the slow dissociation and high affinity. The model also predicts hydrogen bonding between the hydroxyl of Tyr799 and the oxygens of the sulfonyl group of TAK-438. A Tyr799Phe mutation resulted in a 3-fold increase of the dissociation rate, showing that this hydrogen bonding also contributes to the slow dissociation rate. Hence, this K(+)-competitive inhibitor of the gastric H,K-ATPase should provide longer-lasting inhibition of gastric acid secretion compared with previous drugs of this class.
Figures
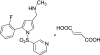
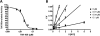
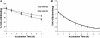
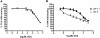
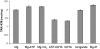


References
-
- Asano S, Yoshida A, Yashiro H, Kobayashi Y, Morisato A, Ogawa H, Takeguchi N, Morii M. (2004) The cavity structure for docking the K(+)-competitive inhibitors in the gastric proton pump. J Biol Chem 279:13968–13975 - PubMed
-
- Berg AL, Böttcher G, Andersson K, Carlsson E, Lindström AK, Huby R, Håkansson H, Skånberg-Wilhelmsson I, Hellmold H. (2008) Early stellate cell activation and veno-occlusive-disease (VOD)-like hepatotoxicity in dogs treated with AR-H047108, an imidazopyridine proton pump inhibitor. Toxicol Pathol 36:727–737 - PubMed
-
- Besancon M, Simon A, Sachs G, Shin JM. (1997) Sites of reaction of the gastric H,K-ATPase with extracytoplasmic thiol reagents. J Biol Chem 272:22438–22446 - PubMed
-
- Chang H, Saccomani G, Rabon E, Schackmann R, Sachs G. (1977) Proton transport by gastric membrane vesicles. Biochim Biophys Acta 464:313–327 - PubMed
-
- Dent J, Kahrilas PJ, Hatlebakk J, Vakil N, Denison H, Franzén S, Lundborg P. (2008) A randomized, comparative trial of a potassium-competitive acid blocker (AZD0865) and esomeprazole for the treatment of patients with nonerosive reflux disease. Am J Gastroenterol 103:20–26 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

