Tsc1 mutant neural stem/progenitor cells exhibit migration deficits and give rise to subependymal lesions in the lateral ventricle
- PMID: 21828270
- PMCID: PMC3182017
- DOI: 10.1101/gad.16750211
Tsc1 mutant neural stem/progenitor cells exhibit migration deficits and give rise to subependymal lesions in the lateral ventricle
Abstract
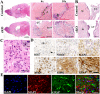
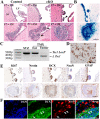
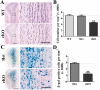
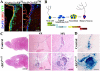
Subependymal nodules (SENs) and subependymal giant cell astrocytomas (SEGAs) are common brain lesions found in patients with tuberous sclerosis complex (TSC). These brain lesions present a mixed glioneuronal phenotype and have been hypothesized to originate from neural stem cells. However, this hypothesis has not been tested empirically. Here, we report that loss of Tsc1 in mouse subventricular zone (SVZ) neural stem/progenitor cells (NSPCs) results in formation of SEN- and SEGA-like structural abnormalities in the lateral ventricle, the consequence of abnormal migration of NSPCs following Tsc1 loss.
Figures
References
-
- Buccoliero AM, Franchi A, Castiglione F, Gheri CF, Mussa F, Giordano F, Genitori L, Taddei GL 2009. Subependymal giant cell astrocytoma (SEGA): is it an astrocytoma? Morphological, immunohistochemical and ultrastructural study. Neuropathology 29: 25–30 - PubMed
-
- Burger PC, Scheithauer BW, Vogel FS 2002. Surgical pathology of the nervous system and its coverings. Churchill Livingstone, New York.
-
- Ess KC, Kamp CA, Tu BP, Gutmann DH 2005. Developmental origin of subependymal giant cell astrocytoma in tuberous sclerosis complex. Neurology 64: 1446–1449 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
