Alteration of pulmonary artery integrin levels in chronic hypoxia and monocrotaline-induced pulmonary hypertension
- PMID: 21829038
- PMCID: PMC3169367
- DOI: 10.1159/000329593
Alteration of pulmonary artery integrin levels in chronic hypoxia and monocrotaline-induced pulmonary hypertension
Abstract
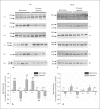
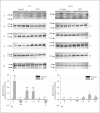
Background: Pulmonary hypertension is associated with vascular remodeling and increased extracellular matrix (ECM) deposition. While the contribution of ECM in vascular remodeling is well documented, the roles played by their receptors, integrins, in pulmonary hypertension have received little attention. Here we characterized the changes of integrin expression in endothelium-denuded pulmonary arteries (PAs) and aorta of chronic hypoxia as well as monocrotaline-treated rats.
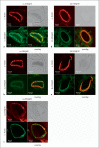
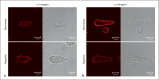
Methods and results: Immunoblot showed increased α(1)-, α(8)- and α(v)-integrins, and decreased α(5)-integrin levels in PAs of both models. β(1)- and β(3)-integrins were reduced in PAs of chronic hypoxia and monocrotaline-treated rats, respectively. Integrin expression in aorta was minimally affected. Differential expression of α(1)- and α(5)-integrins induced by chronic hypoxia was further examined. Immunostaining showed that they were expressed on the surface of PA smooth muscle cells (PASMCs), and their distribution was unaltered by chronic hypoxia. Phosphorylation of focal adhesion kinase was augmented in PAs of chronic hypoxia rats, and in chronic hypoxia PASMCs cultured on the α(1)-ligand collagen IV. Moreover, α(1)-integrin binding hexapeptide GRGDTP elicited an enhanced Ca(2+) response, whereas the response to α(5)-integrin binding peptide GRGDNP was reduced in CH-PASMCs.
Conclusion: Integrins in PASMCs are differentially regulated in pulmonary hypertension, and the dynamic integrin-ECM interactions may contribute to the vascular remodeling accompanying disease progression.
Copyright © 2011 S. Karger AG, Basel.
Figures

Similar articles
-
Integrin ligands mobilize Ca2+ from ryanodine receptor-gated stores and lysosome-related acidic organelles in pulmonary arterial smooth muscle cells.J Biol Chem. 2006 Nov 10;281(45):34312-23. doi: 10.1074/jbc.M606765200. Epub 2006 Sep 9. J Biol Chem. 2006. PMID: 16963791
-
Magnesium Supplementation Attenuates Pulmonary Hypertension via Regulation of Magnesium Transporters.Hypertension. 2021 Feb;77(2):617-631. doi: 10.1161/HYPERTENSIONAHA.120.14909. Epub 2020 Dec 28. Hypertension. 2021. PMID: 33356397
-
Enhanced store-operated Ca²+ entry and TRPC channel expression in pulmonary arteries of monocrotaline-induced pulmonary hypertensive rats.Am J Physiol Cell Physiol. 2012 Jan 1;302(1):C77-87. doi: 10.1152/ajpcell.00247.2011. Epub 2011 Sep 21. Am J Physiol Cell Physiol. 2012. PMID: 21940663 Free PMC article.
-
Pulmonary arterial hypertension: a disease of tethers, SNAREs and SNAPs?Am J Physiol Heart Circ Physiol. 2007 Jul;293(1):H77-85. doi: 10.1152/ajpheart.01386.2006. Epub 2007 Apr 6. Am J Physiol Heart Circ Physiol. 2007. PMID: 17416597 Review.
-
Pulmonary Vascular Remodeling in Pulmonary Hypertension.J Pers Med. 2023 Feb 19;13(2):366. doi: 10.3390/jpm13020366. J Pers Med. 2023. PMID: 36836600 Free PMC article. Review.
Cited by
-
Targeting integrin pathways: mechanisms and advances in therapy.Signal Transduct Target Ther. 2023 Jan 2;8(1):1. doi: 10.1038/s41392-022-01259-6. Signal Transduct Target Ther. 2023. PMID: 36588107 Free PMC article. Review.
-
Mechanobiological Feedback in Pulmonary Vascular Disease.Front Physiol. 2018 Jul 25;9:951. doi: 10.3389/fphys.2018.00951. eCollection 2018. Front Physiol. 2018. PMID: 30090065 Free PMC article. Review.
-
Receptor binding domain of SARS-CoV-2 is a functional αv-integrin agonist.bioRxiv [Preprint]. 2022 Apr 11:2022.04.11.487882. doi: 10.1101/2022.04.11.487882. bioRxiv. 2022. Update in: J Biol Chem. 2023 Mar;299(3):102922. doi: 10.1016/j.jbc.2023.102922. PMID: 35441172 Free PMC article. Updated. Preprint.
-
Role of extracellular matrix in the pathogenesis of pulmonary arterial hypertension.Am J Physiol Heart Circ Physiol. 2018 Nov 1;315(5):H1322-H1331. doi: 10.1152/ajpheart.00136.2018. Epub 2018 Aug 24. Am J Physiol Heart Circ Physiol. 2018. PMID: 30141981 Free PMC article. Review.
-
Pentastatin, a matrikine of the collagen IVα5, is a novel endogenous mediator of pulmonary endothelial dysfunction.Am J Physiol Cell Physiol. 2023 Nov 1;325(5):C1294-C1312. doi: 10.1152/ajpcell.00391.2023. Epub 2023 Sep 11. Am J Physiol Cell Physiol. 2023. PMID: 37694286 Free PMC article.
References
-
- Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:13S–24S. - PubMed
-
- Crouch EC, Parks WC, Rosenbaum JL, Chang D, Whitehouse L, Wu LJ, Stenmark KR, Orton EC, Mecham RP. Regulation of collagen production by medial smooth muscle cells in hypoxic pulmonary hypertension. Am Rev Respir Dis. 1989;140:1045–1051. - PubMed
-
- Durmowicz AG, Stenmark KR. Mechanisms of structural remodeling in chronic pulmonary hypertension. Pediatr Rev. 1999;20:e91–e102. - PubMed
-
- Zaidi SH, You XM, Ciura S, Husain M, Rabinovitch M. Overexpression of the serine elastase inhibitor elafin protects transgenic mice from hypoxic pulmonary hypertension. Circulation. 2002;105:516–521. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

