Rhythmic actomyosin-driven contractions induced by sperm entry predict mammalian embryo viability
- PMID: 21829179
- PMCID: PMC3265380
- DOI: 10.1038/ncomms1424
Rhythmic actomyosin-driven contractions induced by sperm entry predict mammalian embryo viability
Abstract
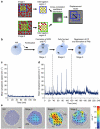
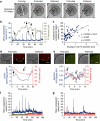
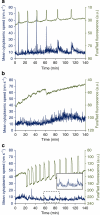
Fertilization-induced cytoplasmic flows are a conserved feature of eggs in many species. However, until now the importance of cytoplasmic flows for the development of mammalian embryos has been unknown. Here, by combining a rapid imaging of the freshly fertilized mouse egg with advanced image analysis based on particle image velocimetry, we show that fertilization induces rhythmical cytoplasmic movements that coincide with pulsations of the protrusion forming above the sperm head. We find that these movements are caused by contractions of the actomyosin cytoskeleton triggered by Ca(2+) oscillations induced by fertilization. Most importantly, the relationship between the movements and the events of egg activation makes it possible to use the movements alone to predict developmental potential of the zygote. In conclusion, this method offers, thus far, the earliest and fastest, non-invasive way to predict the viability of eggs fertilized in vitro and therefore can potentially improve greatly the prospects for IVF treatment.
Conflict of interest statement
A U.S. patent has been filed on this work. 'Methods for predicting mammalian embryo viability' (US application no. 61/503827).
Figures


References
-
- Roegiers F., McDougall A. & Sardet C. The sperm entry point defines the orientation of the calcium-induced contraction wave that directs the first phase of cytoplasmic reorganization in the ascidian egg. Development 121, 3457–3466 (1995). - PubMed
-
- Sardet C., Paix A., Prodon F., Dru P. & Chenevert J. From oocyte to 16-cell stage: cytoplasmic and cortical reorganizations that pattern the ascidian embryo. Dev. Dyn. 236, 1716–1731 (2007). - PubMed
-
- Weaver C. & Kimelman D. Move it or lose it: axis specification in Xenopus. Development 131, 3491–3499 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

