A direct comparison of CellSearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas
- PMID: 21829190
- PMCID: PMC3171010
- DOI: 10.1038/bjc.2011.294
A direct comparison of CellSearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas
Abstract
Background: Circulating tumour cells (CTCs) can provide information on patient prognosis and treatment efficacy. However, there is no universal method to detect CTC currently available. Here, we compared the performance of two CTC detection systems based on the expression of the EpCAM antigen (CellSearch assay) or on cell size (ISET assay).
Methods: Circulating tumour cells were enumerated in 60 patients with metastatic carcinomas of breast, prostate and lung origins using CellSearch according to the manufacturer's protocol and ISET by studying cytomorphology and immunolabelling with anti-cytokeratin or lineage-specific antibodies.
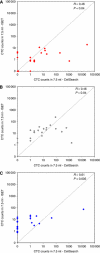
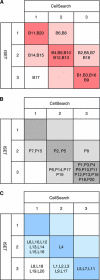
Results: Concordant results were obtained in 55% (11 out of 20) of the patients with breast cancer, in 60% (12 out of 20) of the patients with prostate cancer and in only 20% (4 out of 20) of lung cancer patients.
Conclusion: Our results highlight important discrepancies between the numbers of CTC enumerated by both techniques. These differences depend mostly on the tumour type. These results suggest that technologies limiting CTC capture to EpCAM-positive cells, may present important limitations, especially in patients with metastatic lung carcinoma.
Figures

References
-
- Alix-Panabières C, Riethdorf S, Pantel K (2008) Circulating tumor cells and bone marrow micrometastasis. Clin Cancer Res 14: 5013–5021 - PubMed
-
- Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW (2004) Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 10: 6897–6904 - PubMed
-
- Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen LW, Meropol NJ (2008) Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 26: 3213–3221 - PubMed
-
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351: 781–791 - PubMed
-
- De Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D (2008) Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 14: 6302–6309 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

