Multipotent mesenchymal stromal cells increase tPA expression and concomitantly decrease PAI-1 expression in astrocytes through the sonic hedgehog signaling pathway after stroke (in vitro study)
- PMID: 21829213
- PMCID: PMC3210339
- DOI: 10.1038/jcbfm.2011.116
Multipotent mesenchymal stromal cells increase tPA expression and concomitantly decrease PAI-1 expression in astrocytes through the sonic hedgehog signaling pathway after stroke (in vitro study)
Abstract
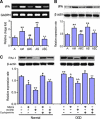
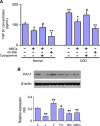
Multipotent mesenchymal stromal cells (MSCs) increase tissue plasminogen activator (tPA) activity in astrocytes of the ischemic boundary zone, leading to increased neurite outgrowth in the brain. To probe the mechanisms that underlie MSC-mediated activation of tPA, we investigated the morphogenetic gene, sonic hedgehog (Shh) pathway. In vitro oxygen and glucose deprivation and coculture of astrocytes and MSCs were used to mimic an in vivo ischemic condition. Both real-time-PCR and western blot showed that MSC coculture significantly increased the Shh level and concomitantly increased tPA and decreased plasminogen activator inhibitor 1 (PAI-1) levels in astrocytes. Inhibiting the Shh signaling pathway with cyclopamine blocked the increase of tPA and the decrease of PAI-1 expression in astrocytes subjected to MSC coculture or recombinant mouse Shh (rm-Shh) treatment. Both MSCs and rm-Shh decreased the transforming growth factor-β1 level in astrocytes, and the Shh pathway inhibitor cyclopamine reversed these decreases. Both Shh-small-interfering RNA (siRNA) and Glil-siRNA downregulated Shh and Gli1 (a key mediator of the Shh transduction pathway) expression in cultured astrocytes and concomitantly decreased tPA expression and increased PAI-1 expression in these astrocytes after MSC or rm-Shh treatment. Our data indicate that MSCs increase astrocytic Shh, which subsequently increases tPA expression and decreases PAI-1 expression after ischemia.
Figures

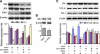
Similar articles
-
Increasing tPA activity in astrocytes induced by multipotent mesenchymal stromal cells facilitate neurite outgrowth after stroke in the mouse.PLoS One. 2010 Feb 3;5(2):e9027. doi: 10.1371/journal.pone.0009027. PLoS One. 2010. PMID: 20140248 Free PMC article.
-
The sonic hedgehog pathway mediates brain plasticity and subsequent functional recovery after bone marrow stromal cell treatment of stroke in mice.J Cereb Blood Flow Metab. 2013 Jul;33(7):1015-24. doi: 10.1038/jcbfm.2013.50. Epub 2013 Apr 3. J Cereb Blood Flow Metab. 2013. PMID: 23549381 Free PMC article.
-
Multipotent mesenchymal stromal cells decrease transforming growth factor β1 expression in microglia/macrophages and down-regulate plasminogen activator inhibitor 1 expression in astrocytes after stroke.Neurosci Lett. 2013 May 10;542:81-6. doi: 10.1016/j.neulet.2013.02.046. Epub 2013 Mar 7. Neurosci Lett. 2013. PMID: 23499476 Free PMC article.
-
Mesenchymal Stromal Cells Promote Axonal Outgrowth Alone and Synergistically with Astrocytes via tPA.PLoS One. 2016 Dec 13;11(12):e0168345. doi: 10.1371/journal.pone.0168345. eCollection 2016. PLoS One. 2016. PMID: 27959956 Free PMC article.
-
Tissue plasminogen activator and plasminogen activator inhibitor 1 contribute to sonic hedgehog-induced in vitro cerebral angiogenesis.PLoS One. 2012;7(3):e33444. doi: 10.1371/journal.pone.0033444. Epub 2012 Mar 14. PLoS One. 2012. PMID: 22432023 Free PMC article.
Cited by
-
The Neuroprotective Roles of Sonic Hedgehog Signaling Pathway in Ischemic Stroke.Neurochem Res. 2018 Dec;43(12):2199-2211. doi: 10.1007/s11064-018-2645-1. Epub 2018 Sep 28. Neurochem Res. 2018. PMID: 30267379 Review.
-
The role of astrocytes in mediating exogenous cell-based restorative therapy for stroke.Glia. 2014 Jan;62(1):1-16. doi: 10.1002/glia.22585. Epub 2013 Nov 4. Glia. 2014. PMID: 24272702 Free PMC article. Review.
-
Subacute intranasal administration of tissue plasminogen activator promotes neuroplasticity and improves functional recovery following traumatic brain injury in rats.PLoS One. 2014 Sep 3;9(9):e106238. doi: 10.1371/journal.pone.0106238. eCollection 2014. PLoS One. 2014. PMID: 25184365 Free PMC article.
-
Stem Cell Recipes of Bone Marrow and Fish: Just What the Stroke Doctors Ordered.Stem Cell Rev Rep. 2017 Apr;13(2):192-197. doi: 10.1007/s12015-016-9716-y. Stem Cell Rev Rep. 2017. PMID: 28064388 Review.
-
The Role of Matrix Metalloproteinases in Hemorrhagic Transformation in the Treatment of Stroke with Tissue Plasminogen Activator.J Pers Med. 2023 Jul 23;13(7):1175. doi: 10.3390/jpm13071175. J Pers Med. 2023. PMID: 37511788 Free PMC article. Review.
References
-
- Alexandre C, Jacinto A, Ingham PW. Transcriptional activation of hedgehog target genes in Drosophila is mediated directly by the cubitus interruptus protein, a member of the GLI family of zinc finger DNA-binding proteins. Genes Dev. 1996;10:2003–2013. - PubMed
-
- Benchenane K, Lopez-Atalaya JP, Fernandez-Monreal M, Touzani O, Vivien D. Equivocal roles of tissue-type plasminogen activator in stroke-induced injury. Trends Neurosci. 2004;27:155–160. - PubMed
-
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001a;32:1005–1011. - PubMed
-
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001b;32:1005–1011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

